- History Home
- People, Leadership & Service
- A Legacy of Excellence
- History & Impact
- Meetings Through the Years
- Resources
Biography - Helen Megaw (1907 - 2002)Biography | Publications | Curriculum Vitae | Videos | Slides | Articles | Obituary
Helen MegawBy Mike Glazer2018
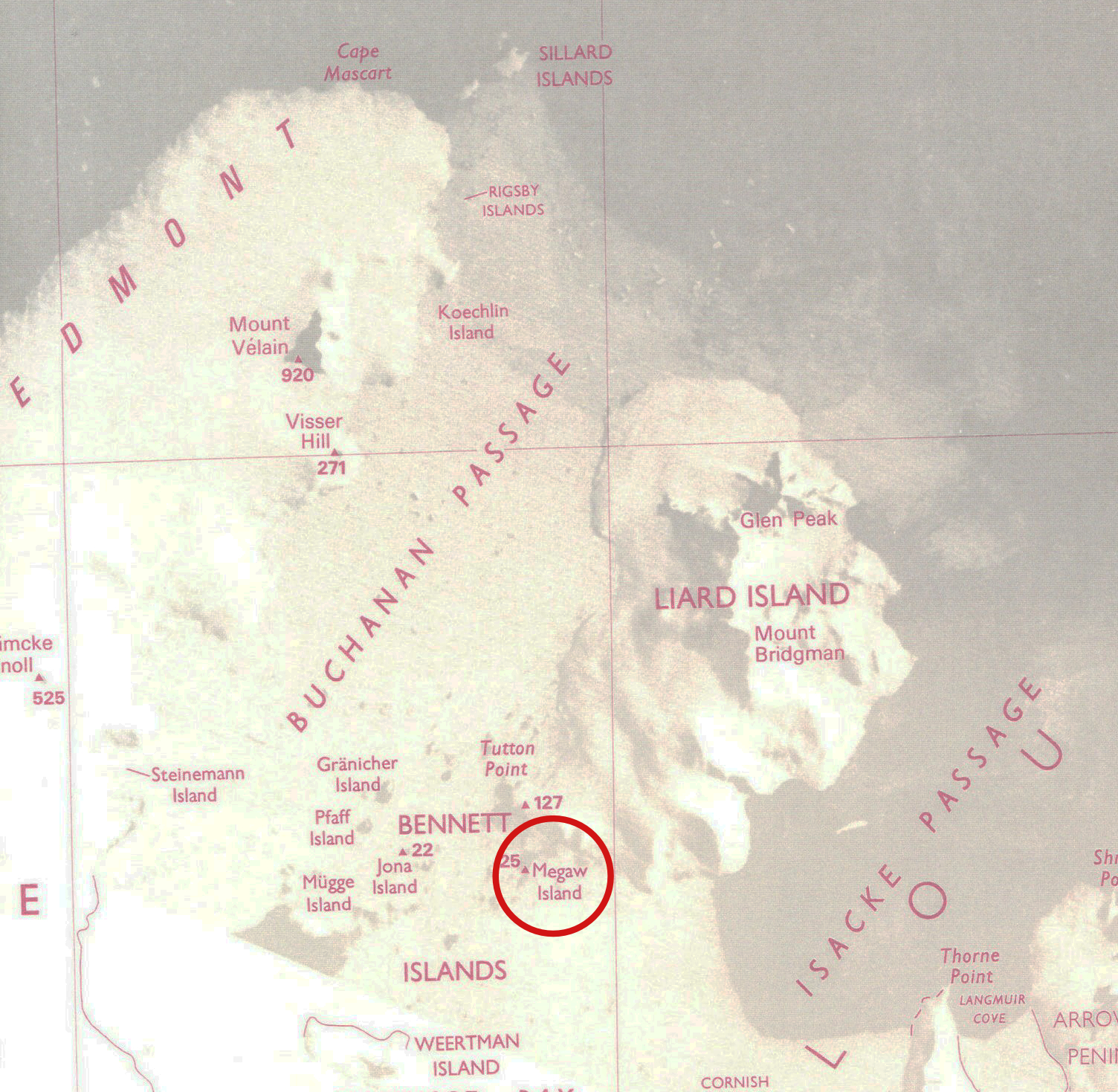
 Helen D. Megaw with her oscillation camera [http://www.newulsterbiography.co.uk/index.php/home/viewPerson/1919]. It was during the IUCr Congress in 1969 at Stony Brook that I first met Helen Dick Megaw. I had been spending a great year in the Chemistry Department at Harvard working in the research group of Jack Gougoutas together with his students Les Lessinger and Jon Clardy. I needed now to consider what I would be doing in the future. I liked the USA very much and was thinking of making my career there. Now, Helen, who was a member of staff at the Cavendish Laboratory, Cambridge, UK, had received some funding to employ a postdoctoral assistant, and my old PhD supervisor, Kathleen Lonsdale, had suggested to her that she should try to persuade me to come and work with her. Helen’s field of interest was in perovskite structures but at that time I was more interested in organic molecules. To be frank, I had never got on well with inorganic chemistry and didn’t think I would ever be able to understand their structures. But Helen was obstinate and kept trying to persuade me to work with her. I think it was at the clambake on Fire Island that I finally gave in and agreed to move back to the UK. I have never regretted that move because I was soon to discover that my “boss” was a very remarkable person from whom I would learn a great deal. So who was Helen Megaw? Well, she was born on June 1st, 1907 into a very distinguished and influential Northern Irish family. Her father, Robert Dick Megaw, was a famous Chancery Judge in the High Court of Justice of Northern Ireland and an Ulster politician. In addition, her uncle, Major-General Sir John Wallace Dick Megaw, was a director of the Indian Medical Service, while one brother built the Mersey tunnel (in Liverpool), the Dartford tunnel (London), the Victoria underground line (London) and Battersea (London) power station. Another brother, Sir John Megaw was a Lord Justice in the Court of Appeal, and one of her sisters researched diet and health in the 1930s and marriage laws in Uganda in the 1950s. A most extraordinary family background. Helen was born in Dublin, Ireland, where she attended the Alexandra School from 1916 until her family moved to Belfast in 1921 just before the partition of Ireland. After a brief period at the Methodist College, Belfast she went to Roedean School, Brighton, England from 1922 until 1925. One of her aunts was secretary to the Mistress of Girton College, Cambridge (one of the only two Cambridge Colleges for women) and Helen's ambition was to study there. She won an exhibition to the College in 1925 but for financial reasons decided to go to Queen's University, Belfast. The next year she won a scholarship and this time it proved possible for her take up a place at Girton. Originally, she had intended to read Mathematics, but she had enjoyed Chemistry at school and on her teacher's advice she opted for Natural Sciences so that she could study both Science and Mathematics. She thought that the regulations required her to study three subjects and she planned to study Chemistry, Physics and Mathematics. However, her Director of Studies, Miss M. B. Thomas, explained that she was required to study three experimental subjects (Mathematics being an optional extra) and she advised Megaw to choose Mineralogy as her third experimental subject. Had Megaw known that she could have chosen Geology instead of Mineralogy she would have opted for Geology and, in all probability, she would not have become a crystallographer! She achieved a Class I in Part I of the Natural Sciences Tripos in 1928. She then specialised in Physics, obtaining a Class II in Part II in 1930. When Ernest Rutherford at the Cavendish Laboratory told her that there was no opportunity for her to do post-graduate work in the Physics department, Miss Thomas suggested that she approach Professor Arthur Hutchinson whose Department of Mineralogy had a strong crystallographic tradition. So it was that she became a research student under the renowned, and some would say infamous, John Desmond Bernal, investigating the thermal expansion of crystals, and the atomic structure of ice and the mineral hydrargillite (a hydroxide of aluminium). One of Bernal’s students at the same time was the young Dorothy Crowfoot, later to become famous as the Nobel Prize winner Dorothy Hodgkin, and Helen and Dorothy became firm friends. Bernal was a stimulating influence on Helen and happily confirmed her interest in crystals. Her choice of Crystallography was a wise one, because it was the one scientific discipline then, thanks principally to W.H. and W.L. Bragg, that had already established itself as a place in which both men and women could engage on an equal basis, and she never, or rarely, was aware of any form of discrimination. She started work on the structure of the mineral hydrargillite, a form of Al(OH)3. In Figure 2 I show a photograph of a model of its structure, which I have in my Crystallography collection in Oxford. Although rather rough, having seen better times, I have kept it because it was her first crystal model and it was constructed with help from Dorothy Hodgkin in 1934.  A model of the crystal structure of hydrargillite constructed in 1934 by Helen Megaw with help from Dorothy (Crowfoot) Hodgkin. Her main Ph.D. work with Bernal was a study of the crystal structures of ice. Helen's accurate and demanding work on ice and heavy ice showed that the hydrogen atoms were involved in bonding between two oxygens. She and Bernal surveyed the known structures of hydroxides and of water, and concluded that there were two types of hydrogen bond. In one type, found in ice, the hydrogen oscillates between two positions, each being closer to one of the oxygen atoms than the other. In the other type the hydrogen is bonded more strongly to one of the two oxygen atoms. In honour of her discoveries of the nature of ice, an island in Antarctica was named Megaw Island in 1962.
Location of Megaw Island in Antarctica 66o55’S, 67o36’W. On another matter, it is often said that it was Bernal who suggested that X-ray photographs of biological crystals could be taken by encapsulating them in a mother-liquor in a glass tube. However, I recall that Helen told me that she had given the idea to Bernal first, as this was the technique that she had already mastered for her work on ice. In 1934 Helen spent a year with Professor Hermann Mark in Vienna and then moved to work briefly under Professor Francis Simon at the Clarendon Laboratory, Oxford. This was followed by two years of school teaching before taking up a position at Philips Lamps Ltd in Mitcham in 1943. It was here that she worked out the crystal structure of a very important industrial material, barium titanate, which is used in capacitors, pressure sensitive devices and in a variety of other electrical and optical applications. This material, which crystallizes in the so-called perovskite structure, belongs to the class of materials known as ferroelectrics, originally discovered around 1935. Because of its strategic military importance much of the work was secret, and Helen was only allowed to publish her work on the structure provided she did not mention its useful properties! This structure is so famous and important that Helen’s name is permanently associated with it and with perovskite structures in general. In the ferroelectrics community, Helen’s contributions are particularly recognised, and her book Ferroelectricity in Crystals published in 1957 was the first of its kind and soon became a classic text. The dust cover of Helen's classic book on ferroelectric crystals. In 1945, she moved to Birkbeck College London, once again to work with Bernal, and in the following year, she was appointed to a post in the Cavendish Laboratory, Cambridge, where she remained for the rest of her scientific life. A second book followed years later entitled Crystal Structures: A Working Approach, a fine text that illustrates well her unique approach to describing the architecture of crystals. Her later detailed studies of the structures and phase transitions in KNbO3, NaNbO3, LiNbO3, and Ca2NbO7, including the change in atomic vibrations near a transition temperature, have contributed much to the understanding of the structural basis of ferroelectricity.  Helen with the image of the perovskite structure for which she is famous. The Cavendish Laboratory was under the leadership of the Nobel Laureate Sir William Lawrence Bragg, and as a result Helen found herself at a place where many important and well-known crystallographers would pass through. She was there during the exciting double-helix days of Crick and Watson and the research by Perutz and Kendrew on haemoglobin and myoglobin. However, she remained loyal to her chosen field of mineralogy and inorganic crystals. Figure 6 shows Helen with some members of staff and students at the Cavendish around 1968.  The Cavendish Laboratory Cambridge ca. 1968. Front row: unknown, A.D. Yoffe, Helen Megaw, Will Taylor, Sir Neville Mott, Jane Brown, Mick Brown. Back students: R. Thornley, unknown, C.N. W. Darlington. In addition to her interest in the structures of ferroelectrics, by suggestion of William Hodge Taylor (often known simply as WHT), her immediate supervisor at the Cavendish Laboratory (Figure 7), she took up an interest in the crystal structures of feldspars. These complicated materials make up most of the earth’s and moon’s surface, and are therefore of great significance in earth sciences. The first structure determination had been carried out by WHT before the war, but such is the complexity of this class of materials, there remained a great deal of unknown science to discover. William Hodge Taylor (WHT) drawn by Elisabeth Vellacott on his retirement. Feldspars are alumino-silicates with the general formula AT4O8 where A is most commonly calcium, sodium or potassium and T represents aluminium and silicon present in the correct proportions to balance the charges on the A ions and the oxygens. Thus, albite has the formula Na(Si3Al)O8, anorthite Ca(Si2Al2)O8andorthoclase, K(Si3Al)O8. The plagioclase feldspars form a chemical series whose formulae may be written Na1+yCay(Si3-yAl1+y)O8 or more simply AnyAb1-y where Ab is albite and An anorthite. Plagioclase compositions appear homogeneous when examined using an optical microscope; there is no evidence of separation into domains of different feldspar phases. However, X-ray diffraction patterns in the composition ranges An30Ab70 to An70Ab30 show diffracted beams, called 'non-Bragg' reflections, that cannot occur in perfectly ordered crystals. Megaw, in three papers published in the Proceedings of the Royal Society in 1960, showed that the arrangement of 'building blocks' in these crystals was disordered. She used diffraction theory to relate the positions and intensities of the non-Bragg reflections to the probability of the occurrence of fault planes in the structure and to the orientation of these planes within the structure. Knowledge of the detailed structural state of a plagioclase specimen is important because it allows the temperature of crystallization and the subsequent thermal history of the rock in which it occurs to be deduced. One of her discoveries in connection with felspars illustrates a special and unique ability. Helen could visualize crystal structures entirely in her head. She never used computers but instead was able to see the atomic arrangements. For instance, if one asked her what a particular structure looked like down a specific direction she would think for a few moments and then draw it! Thus, she identified a special structural feature in felspars involving rigid tilted tetrahedra which she likened to a “crankshaft”. By cooperative tilting this crankshaft can be closed up or extended relatively easily. As a consequence, the distribution of Al and Si in the tetrahedral sites has only a small effect on the bulk moduli of plagioclase felspars. In the years after the war, the economic situation in Britain was dire and recovery was slow. This was a time of much gloom and depression. The government decided that the nation’s spirits would be lifted by holding a special festival in 1951, the Festival of Britain. This was to create a series of country-wide events pointing to the future, with a large exhibition on the South Bank of the Thames in London. (The only remaining building from that exhibition today is the Festival Hall.) Helen had the idea that designs based on Crystallography could be used as decorations throughout the Festival site. She got several crystallographers, including Bragg, Lonsdale and Hodgkin to join in with creating the designs, which were then submitted to the Council of Industrial Design. These were then used in for the textiles and other materials used at the Festival of Britain, including in the foyer of the Regatta Restaurant. The carpets, curtains and table cloths, the knives and forks, glasses and so on – all were decorated with features based on crystal structures. Figure 8 shows one of the popular textiles produced based on Helen’s structure of afwillite and a tie that I possess with Dorothy Hodgkin’s insulin structure. Today, many of these objects are kept at the Victoria and Albert Museum in London; more information can be found in the Art and Design archives at http://www.vam.ac.uk/__data/assets/pdf_file/0015/250125/megaw_aad_1977_03_20141020.pdf. I can strongly recommend the beautiful book From Atoms to Patterns by Lesley Jackson, where the whole story has been recounted with many excellent photographs of the crystallographic designs.
Festival of Britain designs (a) based on Helen's afwillite structure; (b) tie with Dorothy Hodgkin's insulin structure in 1951. In 1958 Helen was invited to Penn State by Ray Pepinsky and she took the opportunity to attend the ACA meeting in Ann Arbor to present a paper on KH2PO4. She reported later, "After it, Pepinsky, in the discussion, made a criticism so fierce that the chairman said he thought I should have the right to reply at the end of the session. Though very grateful to the chairman, I was not seriously worried. I was confident that my own ideas were right, and thought Pepinsky was quite wrong. But I remember that later someone in the audience said to me, “Are you still going to State College?” Well I went to State College, and spent a very enjoyable summer. It was worth the opportunities it gave me to travel, see something of America, meet people and see other labs, rather than for any work I actually did there!" In her last few years at the Cavendish, starting in 1969, she and I worked together, initially on the study of phase transitions in the perovskite NaNbO3. This had a sequence of at least seven different phases depending on temperature. She had already produced a graph of the lattice parameters as a function of temperature based on earlier work, and much of it was guesswork. My job was to build an X-ray camera with a reliable high-temperature system. With this I was able to derive a quite accurate sequence of phase transitions (Figure 9), which in the event was very close to Helen’s original “guesswork”. As a result, I earned Helen’s respect and ensured that we continued to keep in touch even long after she retired. As I explained in an earlier article (in ACA Reflexions, summer, 2017) this led on to my own major contribution to the tilting of octahedra in perovskites. I have always been indebted, therefore, to Helen for pushing me toward trying to understand the geometry of perovskites. Lattice parameters of NaNbO3 as a function of temperature [Glazer & Megaw Acta Cryst. (1973). A29, 489-495]. In 1972 Helen retired to her home in Ballycastle, County Antrim, Northern Ireland (Figure 11) to pursue her other interest, gardening, although she maintained her interest in ferroelectrics and continued to act as a journal referee.
Helen in her garden at Ballycastle with Christine McKie (nee Kelsey), one of Helen's students. In 1989, Helen became the first woman to be awarded the prestigious Roebling Medal of the Mineralogical Society of America. Bob Newnham (Penn State) wrote at the time: "A number of American crystallographer-mineralogists were trained at Cambridge, and all of us remember her meticulous style of teaching symmetry and crystal chemistry. Crystal Structures – A Working Approach, her last book, published in 1973, contains many of the concepts and interesting home problems presented in her classes. She had a kind heart and a patient way with students that cause many of us to look back with great fondness on our days at the Cavendish. Along with Kathleen Lonsdale and Dorothy Hodgkin, Helen Megaw is one of the grand old British school of women crystallographers who serve as role models for many of us – men and women alike. I am proud to have been one of her students."
In 2000 at the age of 93 she was awarded an honorary degree at Queen’s University, Belfast. In a citation for the honorary degree in 2000, Professor Ruth Lynden-Bell recalled those early days of crystallography research: "It is difficult for us to imagine the scientific environment in the nineteen thirties. It was a time of depression with little money and few jobs. Science departments were much smaller and more intimate. X-ray crystallography was a new science which attracted a number of young women such as Dr. Megaw who became distinguished scientists. In those pioneering days preparation of crystals and collection of data was more difficult and more skilled than it is today. Another big difference, which today's graduates may find hard to imagine, was that there were no computers and the tedious and detailed calculations which lead from the brightness of spots on a photographic plate to a three-dimensional crystal structure were all done by hand. Dr. Megaw was one of the pioneers in this field."[http://www.physicshistory.org.uk/women/Pioneers/MHD.htm]. Helen died in Ballycastle on February 26th, 2002 at the great age of 94. Some important publications: "Cell Dimensions of Ordinary and 'Heavy' Ice," Nature134: 900 (1934). "The Function of Hydrogen in Intermolecular Forces," Proceedings of the Royal Society ofLondon A151: 384 (1935), with J.D. Bernal. "Crystal Structure of Barium Titanate," Nature155: 484 (1945). "Origin of Ferroelectricity in Barium Titanate and other Perovskite-type Crystals," Acta Crystallographica5: 739 (1952). "Order and Disorder. I. Theory of Stacking Faults and Diffraction Maxima," Proceedings of the Royal Society ofLondon A259: 59 (1960). "Order and Disorder. II. Theory of Diffraction Effects in the Intermediate Felspars," Proceedings of the Royal Society ofLondon A259: 159 (1960). "Order and Disorder. III. The Structure of the Intermediate Plagioclase Felspars," Proceedings of the Royal Society ofLondon A259: 184 (1960)). "Studies of the Lattice Parameters and Domains in the Phase Transitions of NaNbO3," Acta Crystallographica A29: 489 (1973), with A.M. Glazer. Books Ferroelectricity in Crystals. Methuen, London 1957. Crystallographic Book List. International Union of Crystallography Commission, Utrecht, 1965 (and 2 supplements (1966) (1972)). Crystal Structures: A Working Approach, W.B. Saunders Co., Philadelphia 1973. |