- History Home
- People, Leadership & Service
- A Legacy of Excellence
- History & Impact
- Meetings Through the Years
- Resources
|
Memoir - Celerino Abad-Zapatero Memoir | Publications | Curriculum Vitae | Videos | Slides | Interviews | Articles | Awards | Obituary
Notes of a Protein Crystallographer: From Altar Boy to Sorcerer’s Apprentice
1. Introduction Well before I received the invitation of the ACA historian to write this piece, I had thought of sending an essay with a content similar to this (although shorter) to the editor of Acta Crystallographica Section D. It would have been my last column in the series “Notes of a Protein Crystallographer” with the request to be published posthumously under the title “Notes of a Gone Protein Crystallographer.” I intended to submit the essay to the editors of Acta as my last essay and, hopefully, have it accepted for publication before my final departure. However, the ACA historian managed to send me the invitation before my plans materialized. In this way, I can fortunately write more extensively about my professional and personal life sojourn. I do hope that the content of this essay will be in the archives, and a brief excerpt is published in the ACA RefleXions before my passing. Besides reviewing my modest contributions to the field, it is my intention to provide some personal insights for the younger generations of scientists in general and crystallographers in particular. It is indeed an honor and a privilege to be able to put these notes together to recollect about the history of macromolecular crystallography interlaced with my personal experiences as it relates to the history of the ACA. In addition, it will be a good mental exercise for the younger generations to reflect on what the future of the field might be. This will be the focus of this brief essay. Two complementary sources can be consulted to give the reader a more complete picture of my journey through macromolecular crystallography, interlaced with my life’s journey. First, my modest book Crystals and Life: A Personal Journey (1) with personal anecdotes and reflections. Second, another long essay on the history of macromolecular crystallography with strong autobiographic content was published in 2015 (2); its content is complementary to the narrative presented here. The interested reader is encouraged to consult these two earlier sources for additional details. Specific references to them will be presented along the way to avoid repetition.
2. Contingency of Life In my view, any biographical note should start with a brief reflection on the uncertainty of our own lives and existence. I began these notes on ANZAC Day, April 25, 2015. Among the stories of the families of the survivors there was one that caught my attention. The son of a survivor told the story of how in the heat of battle one of the combatants saw a feeble motion of a hand in an apparently lifeless corpse, which prompted him to tell the paramedics to evacuate that body to a hospital. That contingent instant resulted in the man surviving and being able to go back to his homeland, marry, raise a family, and permit his son to tell the story. Wars do have a significant influence on the history of science, particularly WWI in the 20th century. Some of the best scientific minds of England went to war and never returned, among them the brilliant physicist Henry G. Moseley (1887-1915), soon after his discovery of the law making the frequency of the X-ray emissions a fingerprint of the corresponding atomic element. A dramatic and visually stunning account of the critical actions of WWI was presented in the film Gallipoli (1981). In a different context, I owe my existence to another contingent event related to the Spanish Civil War (1936-1939). It is part of the family folklore that the circumstances of my parents' meeting each other was due to a dramatic event in the years leading to the Spanish Civil war. My father’s family originated from a very modest peasant household in the Castilian region, in the high plateau, central region of Spain, named after the numerous castles built in the Middle Ages as fortresses against the Arabs that occupied Spain from 711 to 1492 A.D. My paternal grandparents made a living by working the dry and poor soil of the Castilian plateau, supplemented by small orchards and vegetable gardens near a tributary of the Douro River, in the minute town of Milagros, near Aranda de Duero in the province of Burgos. There were seven children in the household, and not enough land or resources to allow them to grow to adulthood in the paternal house. Thus, according to tradition, one or several of the siblings was dedicated to the service of the Catholic church, typically, although not necessarily, the one with more aptitude for studies and letters. Thus, my father was sent away at a very young age to a seminary in Catalonia in Northeastern Spain, near the city of Barcelona. An older brother had also emigrated there earlier and was making a living as a modest restaurateur; it appeared to be the natural thing to do. This region of Spain was more industrialized than the backwards, agricultural interior, and there were certainly many more opportunities for the younger generations. Naturally, there was also more social unrest, and the conflicts between the traditional Catholic values of the Spanish society and the industrial forces were more frequent, particularly during the 1930s. During his time at the seminary, my father showed great aptitude for music and in particular organ and choral music. Thus, he did play the organ regularly, and on certain weekends, he was asked to play at certain social events such as weddings and other family celebrations, with the proceeds resulting from this effort going to the seminary funds. The dramatic event that triggered the meeting of my parents is related to my father’s absence from the seminary on a particular weekend. Although the exact details have been difficult to reconstruct and document, during my father’s absence from the community, a group of anticlerical demonstrators entered the seminary and captured the novices; they were either shot or disbanded from the community. My father returned to an empty seminary house, and his personal path was never the same. He left the vocation of the priesthood and returned to his birthplace; in the larger nearby town of Aranda de Duero, he met my mother. She was the daughter of a very lively and intelligent man, part mason, part architect and draughtsman, and indeed a musician in his own right also; I was named after him and always had a special connection with him.
3. Sciences vs. Humanities Based on my father’s background and my grandfather’s inclinations and interests, the atmosphere in the house was dominated by artistic, musical, and literary pursuits. My father was hired by the local parish to teach in the parochial school and also to attend the services of the main Catholic congregation. My mother attended to the four of us, and we lived in a small flat owned by my grandfather. We were certainly not wealthy financially, and my mother had to manage to feed the six of us with a modest allowance, since my father spent a considerable amount of money buying books for his extensive personal library, which contained titles predominantly in the domain of the humanities: literature, art, religion, philosophy, etc. There were always many books in the house, and although we had limited financial resources, I always remember having a very rich intellectual and musical atmosphere. In our household, we celebrated the festivity of St. Cecilia, the patroness of musicians, on November 22 to honor the saint, my father, and my grandfather. Initially (ages 7-10), I went to the parochial school where my father was a teacher, and I was part of the choir of altar boys that sang at the services of the parish daily and especially on Sundays and all Catholic religious holidays. As my father discovered my interest and aptitude for study, he dreamed of his second son being a Dominican scholar. A devoted and ascetic monk, and an intellectual person in the tradition of St. Thomas Aquinas, just to mention a brilliant figure from that tradition, dating back to the Middle Ages.
Figure 1. Corpus-Christi procession in Aranda de Duero, Burgos, Spain, circa 1956. The author is the altar boy located in the center of the first row of three. To the right of the group is his father, Don Juan Abad Barrasús, teacher and choir director. This image reflects the spirit and atmosphere of Spain about two decades after the end of the Civil War (1936-1939).
During the high school years, I did enjoy studying all subjects, browsing, and even reading some of the high-level books in my father’s library. Despite the limited space, my father managed to have a single room devoted to his books and his writing, where he would seclude himself on Sundays to read, take notes, and write. I vividly remember him typing long hours on his rugged mechanical typewriter that I later inherited when I went to college. I learned later that my father sent a weekly column to the regional newspaper (Diario de Burgos) and used most of the income from this activity to buy books and extend his intellectual interests. My mother was not very pleased with arrangements, as she had to struggle with a modest budget to feed all the family. Important also for my education during these years were the ‘excursions’ or trips that my father would organize for the community of parents and students to places related to the history and art of Spain, particularly cathedrals, religious monuments, castles, monasteries, etc. He would charter a local bus and ask for a modest fee from all the participants to pay the expenses. In the summer, we would depart sharply at the announced time in the early morning hours, travel in uncomfortable buses for a few hours, visit the sights, rest for a brief lunch and local sightseeing, and we would return in the evening hours. This modest arrangement made it possible for my father to show us (in the early years, my older brother Juan Gabriel and I) many of the landmark monuments of Spanish history, among others the grandiose monastery of San Lorenzo del Escorial (Fig. 2a), built by Philip II (1556-1598) with the abundant gold of the Americas in the outskirts of Madrid, the newly established capital of the Kingdom of Spain. This unique world architectural landmark is simultaneously a monastery, royal palace, museum, royal pantheon and library and school. In more recent visits, I discovered in the magnificent frescos of the Hall of the Battles that the painters of the sixteenth century had already discovered crystalline arrays (Fig. 2b).
Figure 2. Images from visits to El Escorial ca. 1954. a. My father holding the hands of my brother Juan (left) and me (right) in front of the façade of the Courtyard of the Kings, at the Escorial main entrance. b. Detail of one of the paintings in the Hall of the Battles (‘Sala de las Batallas’), with fresco paintings of the battles of the Spanish Kings against their enemies (ca. 1500s). I rediscovered this detail showing that the painters of the Renaissance had already ‘discovered’ ordered arrays as found in crystals.
In mid-high school (ages 10-17 in the Spanish educational system of the time), I did well in all subjects, including Latin, art history, geography, Spanish literature, and mathematics. I did like to devote time to study and it was truly effortless. I was not particularly interested in physical activities at that time, although I was lucky to have a short but healthy and wiry complexion. When I was barely twelve, I remember that there was a particularly difficult class of mathematics/geometry that was a tremendous barrier for most of my classmates. It was taught by a rather enthusiastic and capable chemist (but he was the math teacher!), from the local sugar factory, where sugar was extracted from abundant sugar beets growing along the banks of the Duero river. His name was D. Antonio Figueras. I do remember studying with great interest for the exam, and amazingly enough, I was one of the few students (two out of about eighty students) who got a perfect grade. This was an important achievement and gave me tremendous confidence. As much as I liked the humanities, I was confident of my mathematical abilities, and I did like the logical reasoning of math. There was no need to memorize many things. A few solid stepping stones were enough to understand and deduce the rest.
Figure 3. Faculty and religious advisors of the Instituto Nacional de Enseñanza media de Aranda de Duero, Academic course, 1959-1960. Significant in my education were Ms. Victoria Serrano (2nd row, fourth from the left), the teacher of the Natural Sciences course, and math teacher and chemist Mr. Antonio Figueras, who taught us mathematics (2nd row, far right).
After completing the academic course at fourteen, there was a very strong summary exam (‘reválida’) that was necessary to pass in order to proceed to the next two courses referred to as “Bachillerato Superior” (Superior Baccalaureate). I also did well and passed. However, the anguish was that after this exam the student had to choose between two alternative and exclusive paths: the sciences or the humanities. I vividly remember sleepless nights and anguished times of reflection as I tried to decide; I couldn’t make up my mind. The humanities choice would be selected for students planning to study law, philosophy, art, etc. The other alternative was chosen by students thinking of becoming engineers, doctors, math teachers or related fields. For me it was not a matter of aptitude or interest; I liked both and I could do well in either of them. I ruminated on this issue for most of the summer months and in the end I matriculated in the science course, much to the chagrin of my father, although he never expressed it openly. Unfortunately, the Spanish society of the time (early 1960’s) did not offer a bright future for their youth in the fields related to the study of the natural sciences, except if one were to follow medicine. Alternatively, the most secure profession of the humanities path was attorney. In either case, my family did not have the financial resources to support my university education and although my father was hopeful that I would continue to college, the financial aspects were still uncertain.
4. Geology, Crystallography and Chemistry vs. Physics I have very fond memories of the two years of Superior Baccalaureate (ages 15-16). I studied very hard, and I was rewarded by what I felt to be an extraordinary expansion of my interests and my perception of the power of the natural sciences to explain the world. I studied physics, chemistry, mathematics, natural sciences and there was room for a course of philosophy and another of art history. These latter two courses were very important for my education because they gave me a wider perspective of human knowledge in general, in contrast to the detailed study of the other subject matters. There was no regret in the fact that I had chosen sciences as my future path. I will mention two recollections of those years, one of which is particularly relevant to the content of these notes. I enjoyed tremendously the trigonometry, calculus with derivatives and some integration,but the most important insight came to me as I discovered the connection between algebra and geometry: analytical geometry, related to algebra and the equations expressed with letters and symbols (i.e., the equation of a circumference), and the representation of those algebraic conditions in the Cartesian plane. How it was possible to find out the equation of a tangent to the circumference (or any analytical curve) at any point in the plane, or any other related manipulations, fascinated me. Ms. Victoria Serrano, the superb teacher of the course of natural sciences, was a critical influence for my future intellectual development. This class was a very comprehensive course that included all the natural sciences with particular emphasis on biology and geology. Within biology, it included botany, human anatomy, some human physiology as well as animal, plant and insect classification. Within geology, it included the study of rocks, minerals, some field geology (although unfortunately, we never had any field trips) and climatology. A very difficult and relatively extensive part of the course included the study and description of many minerals, including chemical composition and distribution. A very important part of the curriculum was an introduction to crystallography so that the different crystal forms of the various minerals could be studied in the appropriate context. At the time, I was not particularly interested in this part of the material, but I understood very well the symmetry groups, crystal forms (holoedric, hemiedric, tetartohedral) and it all seemed so well organized and logical. In addition, there was a cryptic reference to the experiment performed by Laue using crystals to diffract X rays that, although I did not understand, made a significant impact on me. I think that I subconsciously enjoyed all of this, because all those anecdotes are part of my most treasured memories of those years, as I have expressed in some of my other biographical writings (Crystals and Life, 2015) (1). Another inflexion point in my professional career came when I entered the University of Valladolid (Spain) for my undergraduate science education. After a preparatory year and exam (Preuniversitario)we had to matriculate and choose a major from the very first year of college. This was again a difficult choice. Influenced by the role model of the professional Chemist who taught us Math/Geometry in the earlier years, I initially chose Chemistry as a major. It appeared that he was able to make a reasonable living by being a chemist at a local industry and enjoyed teaching, so I thought that I could probably try a similar path. My father secured a loan for the first year of college and later I studied very devotedly to get good grades and obtained a scholarship for the subsequent four college years. This was a tremendous relief for my father. The subconscious avocation about crystals and crystallography appeared again in my first year in college when we had to complete courses in geology and biology, even though they were not strictly part of the major for either chemistry or physics. As an important part of the completion of the classwork in geology we had to assemble cardboard models of the different forms of crystals existing among the various minerals. The final exam consisted of explaining and commenting on any particular form selected by the professor. Building the carboard models, studying and preparing for the exam required an enormous amount of time that I happily spent in my father’s library room with my younger siblings, as opposed to going out with friends. Further details can be found in my previous biographical writings mentioned earlier (1,2). After my first year in college, I decided to change my major to physics. Somehow, the lure of mathematical rigor and the logic of physics was more powerful than the attraction of the analytical chemistry, synthesis or even the more demanding physical chemistry. I did like the combination of strong mathematical content with detailed observations to predict and explain the world around us. I was able to switch with a minimum of logistical problems because just the year before the Faculty of Physics had been started at the University of Valladolid. Otherwise, I would have had to transfer to another university, which would have been rather complicated in the centralized, bureaucratic, educational system of Spain in the 1960s.
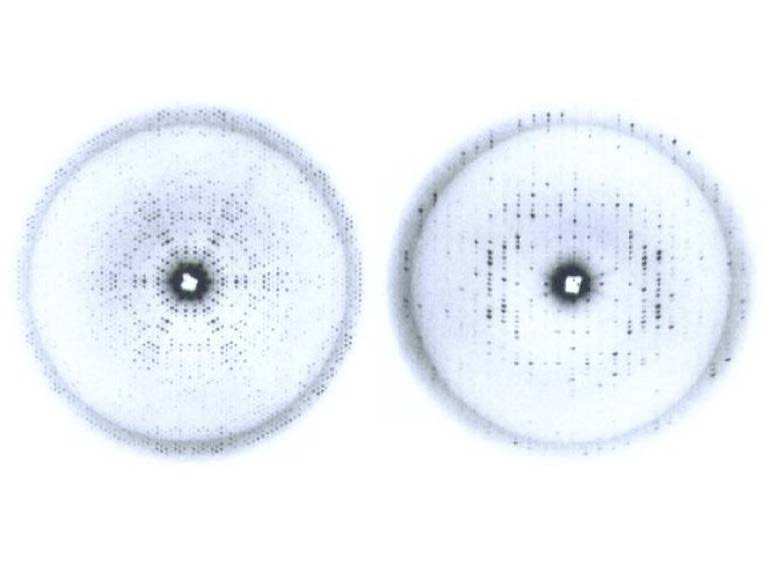
5. Biophysics: Macromolecular Crystallography After five years of college, having a university degree (Licenciado) in physics would allow you to teach at the high school level, in some remote places in Spain, since the positions at the Institutos of Enseñanza Media (equivalent to high schools)of the major Spanish cities (Madrid, Barcelona or even the capital of the provinces) were already occupied by the most senior teachers. I did contemplate this option as a possibility. Positions in industry (or government) were essentially nonexistent, and if available were given to the sons or relatives of influential members of the dominant political force of Spain (Franco’s followers), after the Civil War. However, as a physics major and through the years of study at the university, I had experienced a pulling force towards biological subjects. In the first year of college, I had obtained my best grade in biology, although it was only a minor subject. This interest developed further as I kept on reading articles, material and even news in the press about biological discoveries. I do remember very vividly the photograph of J. Monod, F. Jacob and A. Lwoff at the Pasteur Institute in Paris in the Spanish newspapers when they were awarded the Nobel Prize in Physiology or Medicine in 1965 for their work on the regulation of gene expression (i.e., operon model). I did not know what that was but the images of three French scientists with ties at a press conference for the being scientists caught my attention. The enthusiasm and the vision aside, I needed to find a way to follow my intellectual curiosity and find a way to use my physics training to study some biological project. In retrospect, I found that this interest had also been the driving force behind the migration of many physicists at the end of WWII to study biological processes at the molecular level, notably Max Delbrück and Francis Crick among many of them. I did also read Schrödinger’s inspiring book What is Life?, but I did all these “discoveries” on my own, while in my last undergraduate years. None of my classmates shared these interests and the atmosphere in the physics department where I completed my final courses was not conducive, even hostile, to migrating from physics to biology. I had to find a solution on my own. By accident, I did find an official note at the bulletin board of the University of Valladolid announcing Fullbright Scholarships to pursue graduate studies in the USA. This was a major discovery! After I finished my undergraduate work at the University of Valladolid in 1969, I applied for a scholarship to pursue graduate work in biology at the University of Salamanca, approximately sixty miles away, and I applied for a Fulbright scholarship twice. I failed the first time. I had to take the standard tests required by the American universities at the time (TOEFL, GRE) and entered the bureaucratic labyrinth of the Institute of International Education (IIE). Even taking multiple choice tests with the answer sheet, and number two pencils with erasers were new to me. I had never taken a test using a pencil before; real exams were taken always with a fountain pen or possibly a ballpoint pen. In January 1970, a few weeks after getting married, I vividly remember having to take the bus from Valladolid to Madrid and further away to the American Airforce Base of Torrejón de Ardoz, in the outskirts of Madrid, to take the GRE exams. It was all so American! Scientifically, I was also entering new territory, without knowing it. After the pioneering successes of the globins and the ensuing structures of lysozyme, proteases, and other milestones in the early days of protein crystallography, the field “came of age” in 1971 with the gathering at Cold Spring Harbor Laboratories of the majority of the researchers in the field to tell of their successes. I did not know anything about this. Late in June 1972, my dream was realized. I received a telegram from the Fulbright Office in Madrid saying that I had been offered a scholarship to do graduate work at the University of Texas at Austin, and I needed to make arrangements to start in the fall academic semester. I had married in December 1970, and my dearest young wife, Victoria, and I made plans to travel to the USA in the fall of 1972. Thus, I started my graduate studies at the University of Texas at Austin under the mentorship of Drs. J. Lawrence Fox and Marvin Hackert. The first provided needed advice on coursework, seminars, and basic logistics, and also a beautifully blue purified protein from blue-green algae named phycocyanin (C-phycocyanin, CPC) that I succeeded in crystallizing on my own. The prismatic crystals were taken down to the second floor of Patterson laboratories and offered to Marvin Hackert, a recently hired assistant professor who had worked with Michael Rossmann at Purdue University. With this, my training as a protein crystallographer began, and I saw my professional dream realized: I will be a biophysicist, i.e., a protein crystallographer. The volume published by Cold Spring Harbor Laboratory in 1972 that I saw in Marvin’s office, resulting from the momentous meeting of 1971, was an inspiration for me during my graduate school days. The protein crystallography technology of the time was mainly based on screening and characterization of the crystals by precession photography, followed by data collection using oscillation photography films, processed by the early software programs developed by Michael Rossmann at Purdue. Although I had obtained crystals of two phycobiliproteins (CPC, BPE) diffracting to reasonable resolution for their molecular size, the crystals presented an insidious merohedral twinning that prevented me from making dramatic progress toward the structure solution of either of the two proteins (Figs. 4,5). I collected extensive data sets using oscillation photography of both crystalline proteins and completed an extensive study of their quaternary structures. Certainly, I did learn quite a bit of crystallography, but the final goal of achieving the solution of a novel protein structure eluded us at the time.
Figure 4a. Diffraction images of the blue-green algae C-Phycocyanin from my graduate student days. hk0 (left) and h0l (right) precession photos of the corresponding reciprocal lattice planes.
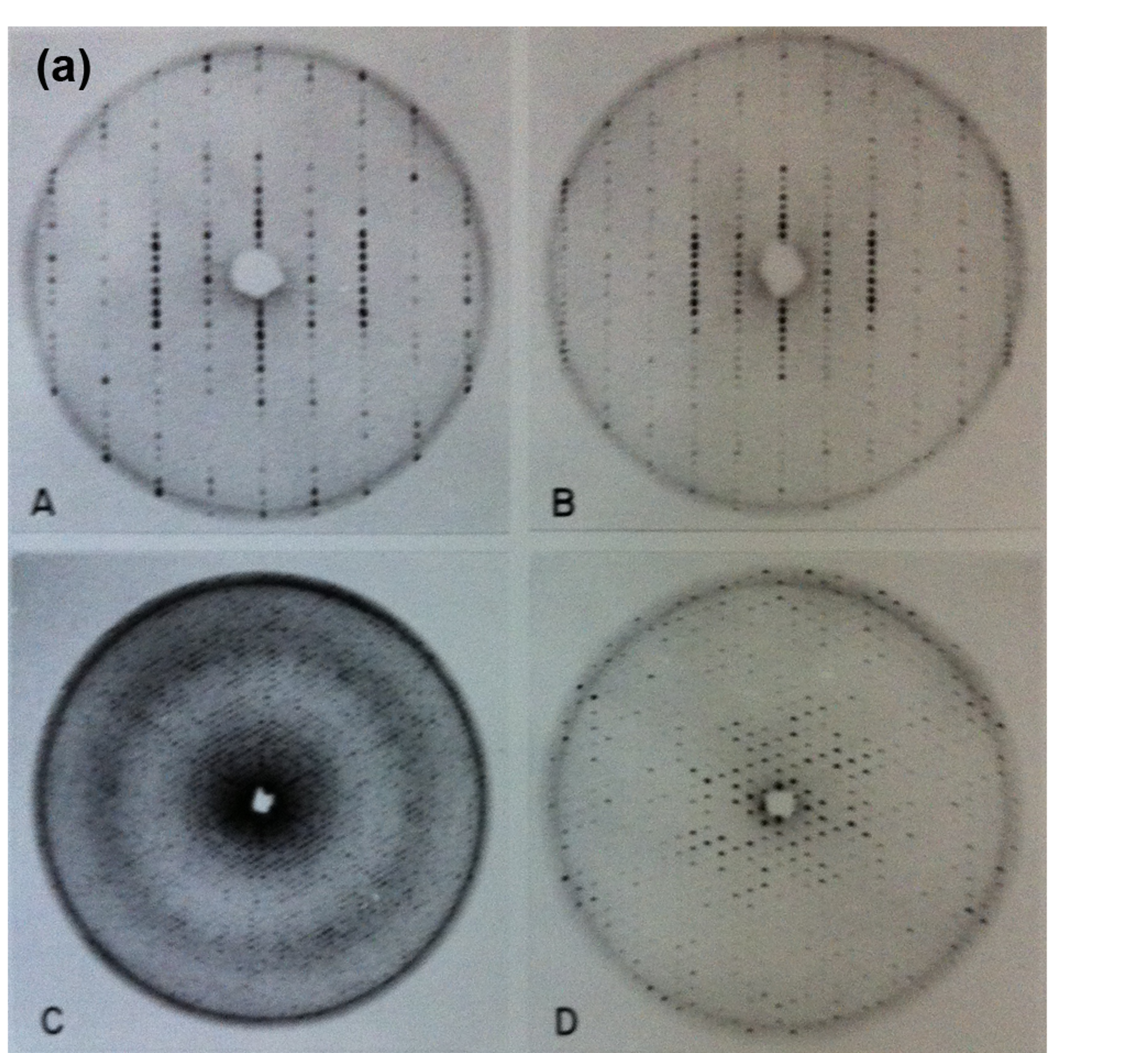
Figure 5a. Diffraction images of the B-Phycoerythrin protein from red-algae. Four different precession photographs A) and B) (upper row) show the hh0 planes for the genuine R3 crystals (left) and the twinned (pseudo R32) crystals. C) and D) are the hk0 (hexagonal) and h0l planes.
Figure 5b. Diffraction images of the B-Phycoerythrin protein from red-algae. An early oscillation diffraction photograph from the same crystals with the hexagonal axis perpendicular to the X-ray beam.
6. Unveiling the Structure of the First Viruses Personally and professionally, the years that I spent at Purdue University as a postdoc were extraordinary. Professionally, I had still much to learn, and the adventure of solving the structure of the first icosahedral (or isometric, as opposed to fibrous) viruses was the frontier of macromolecular crystallography at the time. Personally, the international and unique nature of the crystallography group led by Michael Rossmann was multifaceted and stimulating. I have already described the atmosphere of those amazing and inspiring years in the two sources mentioned before. A brief summary will suffice here. In the late 1970’s the frontier of macromolecular crystallography was to obtain the first atomic structure of the viruses, and in particular the class of viruses described as spherical or isometric viruses, as opposed to the long, rod-like, helicoidal ones like tobacco mosaic virus (TMV) that had already played a critical role in the birth of molecular biology. An extensive discussion of the issues raised by the “crystallization” of TMV by W.M. Stanley and my personal connection to these events has been presented in my other personal recollections (2). As had been suggested by Michael Rossmann, the presence of non-crystallographic symmetry (NCS) in the early crystals obtained from these viruses played a critical role in solving their structures using the MIR method that had been so successful with the globins. There were three groups pursuing three different viruses. The most advanced and best characterized was tomato bushy stunt virus (TBSV) in the group of Stephen Harrison at Harvard. Michael Rossmann had decided to pursue the structure of southern bean mosaic virus (SBMV) at Purdue University. Finally, the smallest satellite tobacco necrosis virus (STNV) was being studied by Bror Strandberg in Uppsala. In addition to the large amount of data that needed to be collected to solve these structures, plus the difficulties of obtaining heavy-atom isomorphous derivatives, the major issue was the design and development of algorithms to average over the NCS of the crystals. The electron density maps without the averaging were too noisy and uninterpretable. TBSV was solved first (3), followed by SBMV (4) and trailed by STNV (5). The most striking result of these studies was that the capsid proteins that enclosed the RNA of these viruses had all the same “jelly-roll” fold (Fig.6). The structural and evolutionary implications of this insight were enormous.
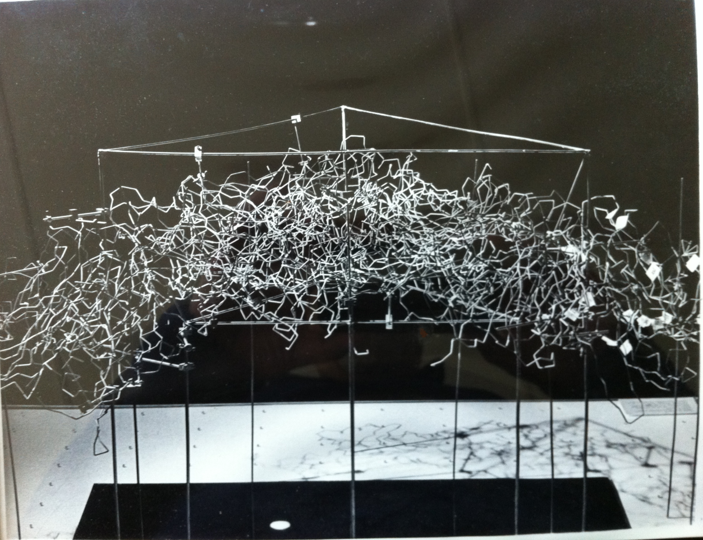
In the best tradition of building mechanical models of biological systems, I spent a considerable amount of time building a wire model of the capsid of SBMV that was extensively studied later by John Erickson for his virus assembly work (Fig. 7). John was a critical player in the astounding success of achieving the structure of the first animal virus (rhinovirus) only a few years later (1985).
Figure 7. Wire model rendering of the protein capsid of SBMV. The model was constructed by assembling a significant number (pentamers-quasi-hexamers) of capsid proteins of the virus created with wire Ca models of the various polypeptide chains (A, B, C). The wire Ca models were produced using the then popular wire-bender, using the bending angles derived from the atomic coordinates. Somehow, the achievement of this group of devoted postdocs and colleagues awoke my poetic muse and inspired me to write the lyrics of a ballad, relating the events and the unexpected findings as a small homage to all of the participants. I borrowed the melody from a song that I heard one day on the radio sung by the inimitable Pete Seeger as I was walking to the lab. The Ballad of the 2.8 Å Structure of SBMV was sung at a party celebration organized by Michael and Aud Rossmann at her house on Nov. 21, 1979. (1, Chapter 22). The climax of the effort and structural insight was summarized in two stanzas of the ballad that read: Eight years have already passed After so many years of labor The full text of the ballad was published in an issue of the bulletin of the Spanish Society of Virology (SEV) in 2013, following the kind invitation of one of the editors (Dr. Carlos Briones Llorente). A scientific achievement inspired an artistic outburst in one of the participants.
7. Refining the Classics The new generations do not realize that even in the early 1980s refining a protein structure of medium to large size (150-300 amino acids) was a challenging feat manually as well as computationally. For about two decades after the first protein structures were solved (myoglobin, hemoglobin), protein structures were built using mechanical parts, typically using Kendrew parts provided by an obscure company in Cambridge, UK named Cambridge Repetition Engineers (Fig. 8a). The importance of mechanical models in crystallography, in general and structural biology in particular has been discussed very extensively and insightfully by Soraya de Chadarevian (6). There are also many other themes related to the origin and development of structural and molecular biology (6).
Figure 8a. The wire model of LDH built in the late sixties at Purdue University using ‘Kendrew’ atomic parts fabricated by Cambridge Repetition Engineers. This represents the technology of the time to build protein structures based on electron density maps drawn on glass windows using a Richard’s Box (also referred to as Richard’s Folly). NAD is bound in the foreground of the model (yellow yarn, middle right), corresponding to the carboxy-end of the NAD-binding domain. Lower right, the simplified depiction of the structure, using a rendering common in the literature in the 1980s. The x-y grid used to measure the coordinates is visible on the floor of the box underneath the model, where the metal rods are screwed in. The z coordinate is the height from the plane of the base, measured with a plumb line. P, Q, R indicate the three orthogonal 2-fold axes. The insert panel shows the Jane Richardson type of diagram of the polypeptide fold drawn by Audrey Rossmann, and the fish drawing (by the author) shows the arrangement of each monomer within the M4 LDH tetramer. The tail of the fish mimics the extension at the amino terminal end that can be seen clearly in the insert and in the computer rendition in Figure 9. The spine and the dorsal fin of the fish are drawn approximately in the direction of the β-strands (Reprinted from J. Mol. Biology. Copyright J. Mol. Biology (8).
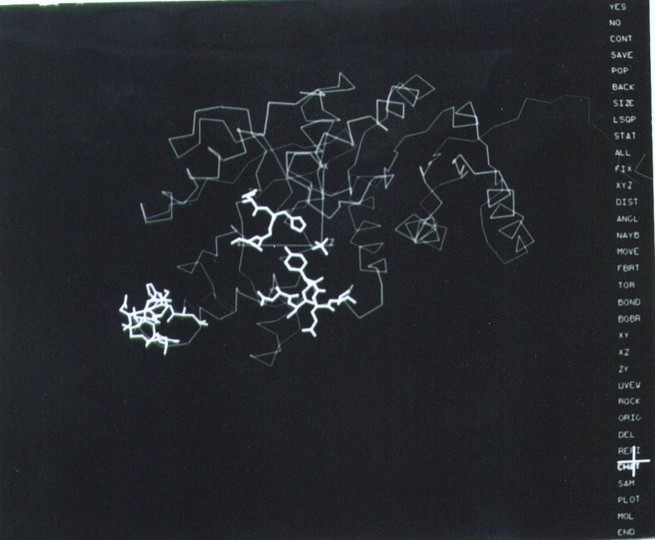
Figure 8b. The revolutionary layout of the computer graphics screen in the early versions of FRODO (1980s). The options are presented on a menu on the right side of the screen and a cursor pen on a tablet (highlighted cross) was used to select them. The image corresponds to the tracing of the refined LDH structure (Fig. 8a), with certain residues and a sulfate ion (center) highlighted in thick lines. Refined coordinates could be saved with the stroke of a pen.
After the work on virus structures, I stayed for a few more years at Michael’s lab taking on the challenge of refining one of the largest protein structures that was solved in the “golden age” of protein crystallography, lactic dehydrogenase (LDH), the iconic structure of the ‘Rossmann fold’. Another large structure, beef liver catalase (BLC), had been solved at the time in the lab and there was much work to do. A collaboration of Michael Rossmann with Joel Sussman (Weizman Institute) and T.A. Jones (University of Uppsala, Sweden) proposed to explore new computational methods and pioneering computer graphics to do the work, and the attraction was irresistible. Sussman had developed the refinement program CORELS (Constraint-Restraint Least Squares) and Jones had created the remarkable computer graphics program FRODO, both specially designed to build and refine protein structures in the computer, superseding the mechanical models (7). The computer and hardware installations for both computational resources were unique, and I had to spend a significant amount of time in each laboratory to achieve significant results in a very short time. I departed from Purdue in the Spring of 1981 to work first with Alwyn Jones in Uppsala and later with Joel Sussman at the Weizman Institute. Within a few intense months of work, in the spring-summer of 1981, I managed to decrease the R-factor of the early LDH wire model from 0.45 to 0.24 with a dramatic improvement on the stereochemical parameters of the model. Similarly dramatic results were obtained soon thereafter with the refinement of BLC at 2.5 A at Purdue, with the new version of FRODO installed locally in the MMSX hardware (Fig. 8b) and the restrained-refinement program PROLSQ developed by Hendrickson and Konnert (1980). Proudly, the refined structure of lactic dehydrogenase was published in 1987 (8), soon after my departure from Michael’s lab for my incorporation to Abbott Laboratories. The refinement of protein structures, even the larger ones, was a laborious but feasible endeavor after those years. In addition to these professionally exciting years, our two dearest children, Inés and Pablo, were born during our stay at Purdue University.
8. Adventures in Structure-Based Drug Design After my postdoctoral years, many of the initial academic positions for protein crystallographers had been filled and it was necessary to find a career path in the early protein crystallography laboratories being set up in the pharmaceutical industry, in the context of what was then called “rational drug design”. This condescending title was later made more acceptable as “structure-guided” and this area of research is now recognized as “structure-based drug design” (SBDD) (Fig. 9).
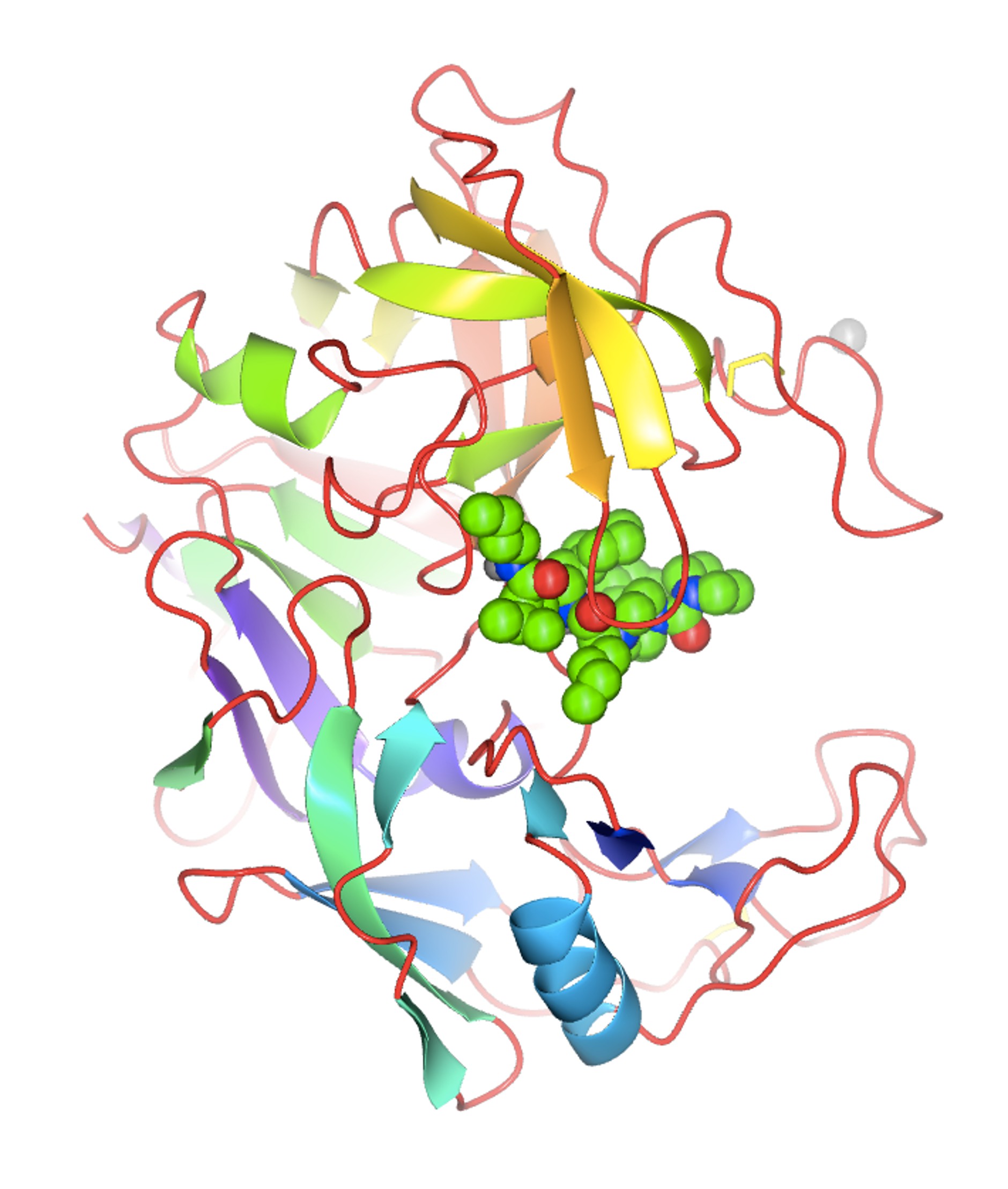
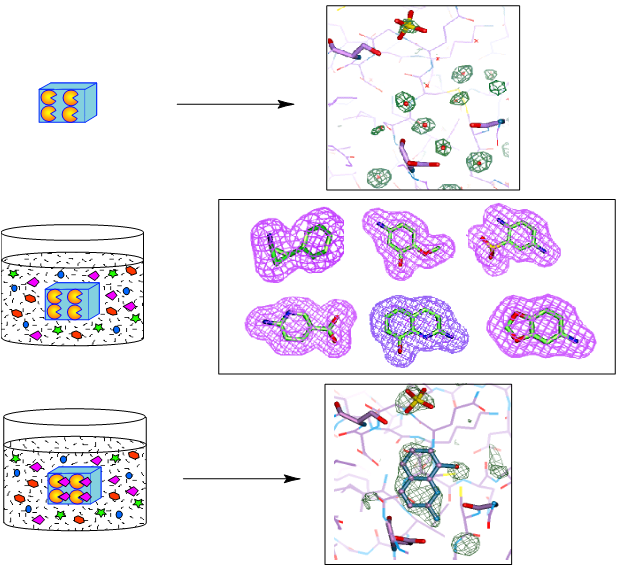
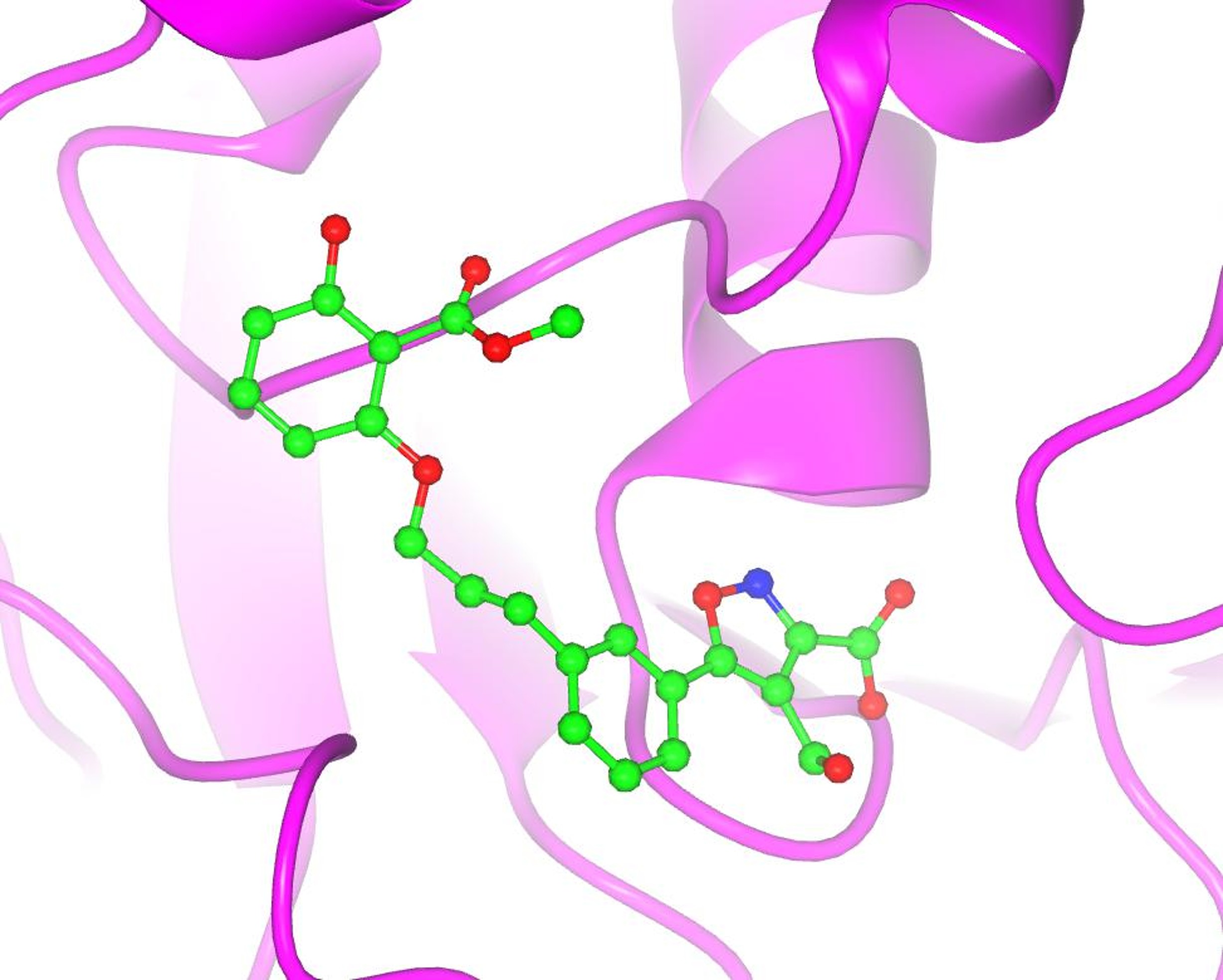
Figure 9. A visual impact of the application of protein crystallography to Structure-Based Drug Design. The structure of the secreted aspartic protease from Candida albicans (SAP2) complexed with an inhibitor designed at Abbott Laboratories in the early 1990s (PDB entry: 1zap). Irrespective of the name, the notion that structural knowledge of the interactions between the ligand (i.e., a small chemical entity) and the target (i.e., the important macromolecule involved in some important biological action) would expedite the design of medicinal drugs seemed logical. There was a flurry of activity in the major pharmaceutical companies setting up laboratories to explore (and possibly exploit) this new technological development. Merck, Monsanto, and Pfizer were among the pioneers, and soon companies like Agouron were established with the premise of using predominantly (if not only!) SBDD methods to design and synthesize active pharmacological entities rapidly. The laboratory of J. Kraut published the first structure of a drug (methotrexate) complexed with a bacterial target in 1978, and it was deposited in the Protein Data Bank (PDB entry 3DFR) in 1982 (9). Much has been published about the use of SBDD in specific therapeutic areas, projects, and targets (see, for instance Charlie Bugg’s Living History memoir). My narrative will be more directed towards the advances in methodology developed in the pharmaceutical industry laboratories during my professional life at Abbott Laboratories. Although I was an academic at heart, visa issues, family responsibilities, and the inability of finding an academic position in Spain (2) made me look for an opportunity in the pharmaceutical laboratories. Luckily, I managed to find a promising position at Abbott Laboratories in the northern suburbs of Chicago. We moved in the spring of 1985. 8.1. Building a laboratory in the pharma industry: the early projects There was an immense sense of optimism in those years among all of us protein crystallographers in the pharmaceutical industry. There was no need to write lengthy grant proposals to secure funding to get the necessary hardware, software and supporting services to build a successful protein crystallography lab. Often, our labs were connected either administratively or up the managerial link to molecular modeling groups or computational chemistry departments. The notion was that our results will feed into the molecular modeling being done by the computational chemists and feed back into the medicinal chemists, allowing the design of “intelligent” compounds faster. At the beginning, though, we faced the difficult and time-consuming nature of our methodology. The structure solution of novel target proteins by MIR methods or even the refinement of existing structures for relevant biological targets of interest was very time consuming. The Protein Data Bank had not reached a significant coverage of folds or structures of many proteins of biomedical interest (particularly from humans or from important pathogens). Crystals diffracting at high resolution were difficult to obtain, and the collection of data for proteins was still done with local, in-house X-ray sources. The chemical ingenuity and the expediency of the molecular modelers beat our results, often by months. It was difficult to have a timely input into the projects to convince our management that certain important compounds (within a series, or a lead compound for further development) were designed by SBDD. Even within our own laboratory, we recognized that the results from our structure-based methods had a rather limited impact on the outcome of successful projects in the clinic. The input of crystallography or crystallographers was not recognized in the filings of significant patents. However, there was one program where the SBDD technology had an important effect because the crystallographic methods could provide some important results in a timely manner. This was partly due to the fortunate circumstance that a critical enzyme in the reproductive cycle of the HIV virus was an aspartic protease. This family of enzymes, starting from the pepsin of the early work of J.D. Bernal, had a long tradition in crystallography and was also an important target for the design of anti-hypertension agents interacting with renin, a structural relative of pepsin. There was a long tradition of active compounds against this class of enzymes at Abbott Labs, and by an uneven mixture of SBDD and chemical ingenuity within the team of medicinal chemists, Abbott Laboratories managed to market important compounds against the AIDS virus: Norvir, Ritonavir, and later Lopinavir. The pioneering work on this target at Abbott Laboratories was done by my colleague, Dr. John Erickson, while I worked in setting up some of the software tools needed for crystallography, including crystallographic refinement. We were hired at the same time to set up the laboratory, and by common agreement, we divided the effort that way. He was the one who designed and pursued the early C2 symmetrical inhibitors based on the dimeric structure of the small HIV protease (10). In the early years, much of the success in SBDD in the pharmaceutical industry at large was related to these compounds and many others in various industrial laboratories. The detailed analysis by crystallography of the subsequent compounds was done by later members of the laboratory, in particular Dr. Chang Park and, later, Dr. Vincent Stoll. All these efforts gave our group and the various SBDD groups in the pharmaceutical industry confidence in our methods and established the role that we could play in helping the drug-discovery effort world worldwide. 8.2. Access to synchrotrons by the pharma industry These early achievements aside, a combination of technical developments in the field and sociological changes in the pharmaceutical industry transformed the impact of SBDD in drug discovery. I do not have the space to discuss the impact of DNA recombinant technology, protein expression, and protein purification in providing substantial amounts of material of critical biomedical targets. I will focus on the developments related to the physical sciences. The development of synchrotron X-ray sources and the routine access by the protein crystallography teams from industry to these technologies changed the modus operandi of SBDD inside the pharma labs and the perception outside. The technology came from the physicists and who had realized in the 1940s that particle accelerators produced an ‘unwanted’ electromagnetic radiation, typically in the X-ray part of the spectrum. Protein crystallography stations were soon set up in synchrotron installations around the world at the National Synchrotron Light Source in Brookhaven National Laboratory, CHESS at Cornell, Daresbury in the UK, and others. Storage rings designed purposely to optimize the production of this type of radiation were built beginning in the mid-1980s, among them the ESRF in Grenoble, SPring-8 in Sayo, Japan, and others. Highly significant for our laboratory at Abbott was the decision to build a third-generation synchrotron (Advanced Photon Source) at Argonne National Laboratory in the western suburbs of Chicago, within driving distance from the lab. I have published a ‘Notes’ essay as a small homage to the pioneers (Gerd Rosenbaum) of this development in our field, which took place initially in Hamburg (Germany), driven by the interest in studying muscle contraction by Kent Holmes. The interested reader is referred to that detailed discussion (11). I wish to emphasize here the role of the instrument makers that made this technology possible. Not being a mechanically minded person, I wish to emphasize here the tremendous importance of technological advances such as synchrotron radiation, detector design, remote and engineering controls (see below) in our field. In addition, there was a very important sociological innovation in the pharmaceutical industry that allowed the use and exploitation of these technological resources to the fullest. In the early years of access to synchrotron sources, most of the allotted time was given to high-reputation academic laboratories with cutting-edge projects. I can mention the work on the rhinovirus structure being done by Michael Rossmann and his team (including John Erickson, Eddy Arnold). These high-profile projects were important to secure the funding and reputation of the early synchrotron beamlines and stations. In contrast, the projects presented by the pharmaceutical industry were not high-profile. They typically required the routine data collection of multiple target-ligand complexes, with very well-established crystallographic parameters, to obtain routine electron density maps. The results required the analyses of relatively small differences in the conformation or structure of the target-ligand pairs. The higher resolution obtained and the speed of data collection were certainly valuable to the projects, but these factors did not weigh heavily in the ranking of the projects requesting beam time. There was also the issue of the privacy of the data collected at government-owned X-ray sources and its impact on the patentability of the resulting chemical matter. This was a major concern for the industry's legal departments. Faced with those issues, the pharmaceutical industry members concluded that they needed independent, routine access to synchrotron radiation sources to expedite the application of SBDD to drug discovery projects in a timely manner, with the appropriate legal framework to protect the inventions derived from the collected data. This was the notion and concept behind the formation of the Industrial Macromolecular Crystallography Association (IMCA), a consortium of several pharmaceutical companies to design, build and equip beamline stations at the APS for use in their drug discovery projects. The management and legal departments of the pharmaceutical giants struggled with the notion of sharing a resource with their competitors; it was unprecedented. However, in the end, the respective teams of protein crystallographers argued that it was imperative to proceed that way if the new methodology was to be used effectively. Through the years, the IMCA consortium consisted of 12 initial companies and has operated two beamlines at sector 17 at the APS. Although the names of the participants have changed due to mergers and acquisitions, the stations have allowed routine access to excellent crystallographic data and resources, within a legal framework acceptable to the pharmaceutical industry. The effort to set up the consortium and build the IMCA beamlines was collective, spanning from the late 1980s to the 1990s, but I do feel proud that I was an important part of it. This changed the modus operandi of SBDD in the pharmaceutical industry. The names of several other colleagues from the pharmaceutical industry, such as Keith Watenpaugh, Bill Stallings, Noel Jones, among others, should also be acknowledged here as early drivers and participants in the IMCA consortium. This was critical. The possibility of obtaining high-quality data rapidly, and with the parallel developments of high-performance computer graphics, reliable refinement algorithms, and superior software tools, as well as the expansion of the available structures in the PDB, protein crystallographers could provide rapid structural responses to drug discovery projects. Quite often, it was now possible to beat the computer modelers to address medicinal chemistry questions related to series optimization based on experimental results, not hypothetical models. It was also possible to tackle the structure determination of more challenging targets with new methods, and with the help of new members of the laboratory, Drs. S. Muchmore, D. Bussiere, and C. Dealwis, we solved the structure of ErmC´ (12), an important structure in the mechanisms of erythromycin resistance in bacterial, using NSLS synchrotron source and the new Se-Met method pioneered by Wayne Hendrickson and coworkers. A higher resolution structure of ErmC´ was later solved and refined in our laboratory by Dr. Gerd Schluckebier, an extremely competent, hardworking, and pleasant colleague who was at the lab for a few years as a postdoctoral associate. 8.3. Crystallographic screening and ACTOR. In the late 1990’s, protein crystallography was confidently settled in the drug discovery arena. It was a time of expansion and self-confidence in the pharmaceutical industry. But with the confidence came the drive to innovate and expand the limits of what crystallography could do to impact drug discovery in different ways. From the very beginning of SBDD it was clear that protein crystallography could be of great value in the optimization process. It could provide very valuable information on the target-ligand interactions and quite often provide very valuable and important suggestions on how to improve the potency of the compounds by optimizing those interactions. However, managers and directors were pushing our lab to expand our role to other stages of the drug discovery process. Another new member of the laboratory, Dr. V. Nienaber, played a critical role in expanding the role of crystallography to the screening of limited mixtures of small compounds (typically 8-10) using “shape-diverse” libraries using the pre-grown crystals of the target (13). The shape diversity of the ligands included in the small mixtures was critical to facilitate the interpretation of the resulting electron density maps (Fig. 10).
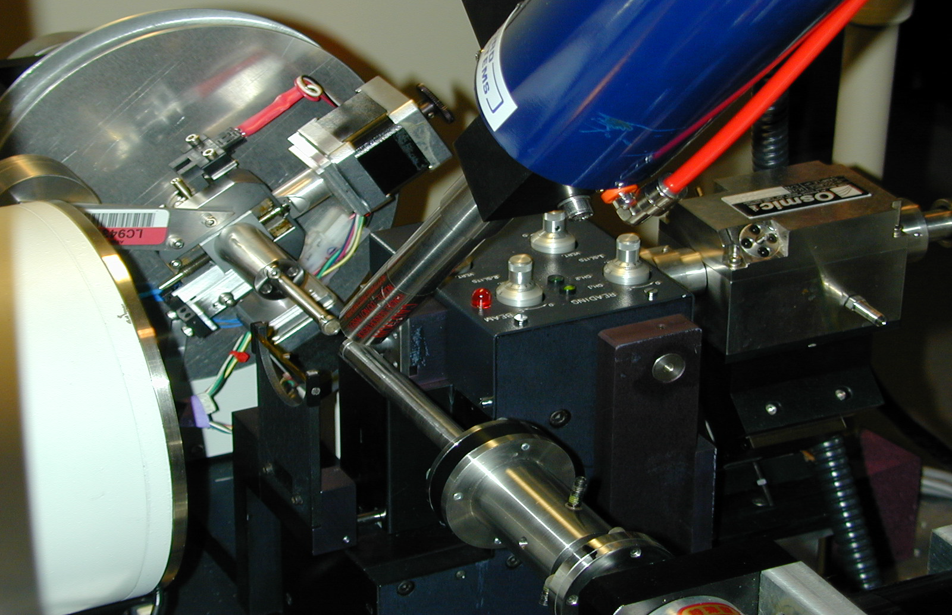
Unbeknown to us, a similar approach had already been explored by Verlinde and Hol in 1994 and so the idea was not particularly novel or unique. However, the idea of screening compounds using crystals presented the challenge of how to develop methods for higher throughput crystallography using conventional (in house) X-ray sources. This problem was taken as a major engineering challenge by the automation department at Abbott Labs and with the total involvement of Jeff Olson, Ron Jones, Jeff Pan, Michael Blum and other collaborators, they addressed the problem of automatically mounting, centering, and exposing the frozen target crystals. From the crystallography side, Drs. Muchmore and Nienaber drove the project, showing the engineers how the protein crystals were frozen, mounted and exposed to X-rays using our rotating anode source. All these concepts formed the basis of the prototype design that was first demonstrated in the Automation Engineering Laboratory at Abbott in July 1998. The first successful operation resulting in good diffraction data took place in the Protein Crystallography Laboratory around September of the same year and the first round-the-clock run with 18 crystals successfully analyzed over a 42-hour run was in November 1998. The achievement was presented at the ACA Meeting in July 2000 (Fig. 11). The technology was licensed to Rigaku/MSC in May, 2001 and the commercial implementation of ACTOR (Automated Crystal Transport Orientation and Retrieval) received an R&D 100 Award for 2002 (Fig 11c. Oxford museum-ACTOR). Other engineering implementations of similar technology are now available at various synchrotron beamlines for protein crystallography all over the world.
Figure 11. Instrumental setup of the crystal mounting robot at cryo-temperatures developed at Abbott Laboratories. a. Overall view with the mounting robot arm (Yamaha device, right), with the extended arm. Lower center is the crystal depository maintained in liquid nitrogen). Left, the commercial ‘MAR’ area detector that was the standard detector for in-house data collection. b. Close-up of the set up: mounting arm in the center, cryo-nozzle at the top, and the servo motors on the back plate to allow the automatic centering of the crystal. c. The commercial ACTOR robot manufactured by Rigaku that became popular at synchrotron beamlines. The latter photo by the author at the Oxford Museum of Science in the UK. All other images from my personal collection.
8.4. Fragment-based drug design At the beginning of my tenure at Abbott labs the approaches to find new promising compounds for further development in the pharmaceutical industry had changed or were changing very rapidly. The extensive natural products research unit at Abbott was dismantled; the notion and concept of screening the existing large collection of compounds seeking promising compounds for other therapeutic targets was successfully implemented. This approach was routinely used for many new projects. And yet, the extensive use of large proprietary libraries to screen for suitable leads for new targets had its drawbacks. Quite often, the active compounds were rather large, and the scaffolds only allowed limited chemistry to optimize the interactions, even using the SBDD methodology. In addition, frequently the active compounds had, from the start, rather unsuitable pharmaco-kinetic properties, making it rather difficult to redirect the chemistry to more favorable scaffolds. Our X-ray laboratory and our colleagues at the NMR group at Abbott, as well as other labs in the community, thought about using smaller molecules (or “fragments”) as a more promising way out of this problem. This concept drove the crystallographic screening and robot development in our environment. The NMR lab at Abbott, headed by Dr. Steve Fesik, also explored this idea in its own way and provided a dramatic proof of concept that was published in Science in 1996 (14). The method was nicknamed “SAR by NMR” and was based on the screening and linking of small fragment libraries, using the NMR spectra to monitor the interactions. The ideal strategy is sketched in Fig. 12. 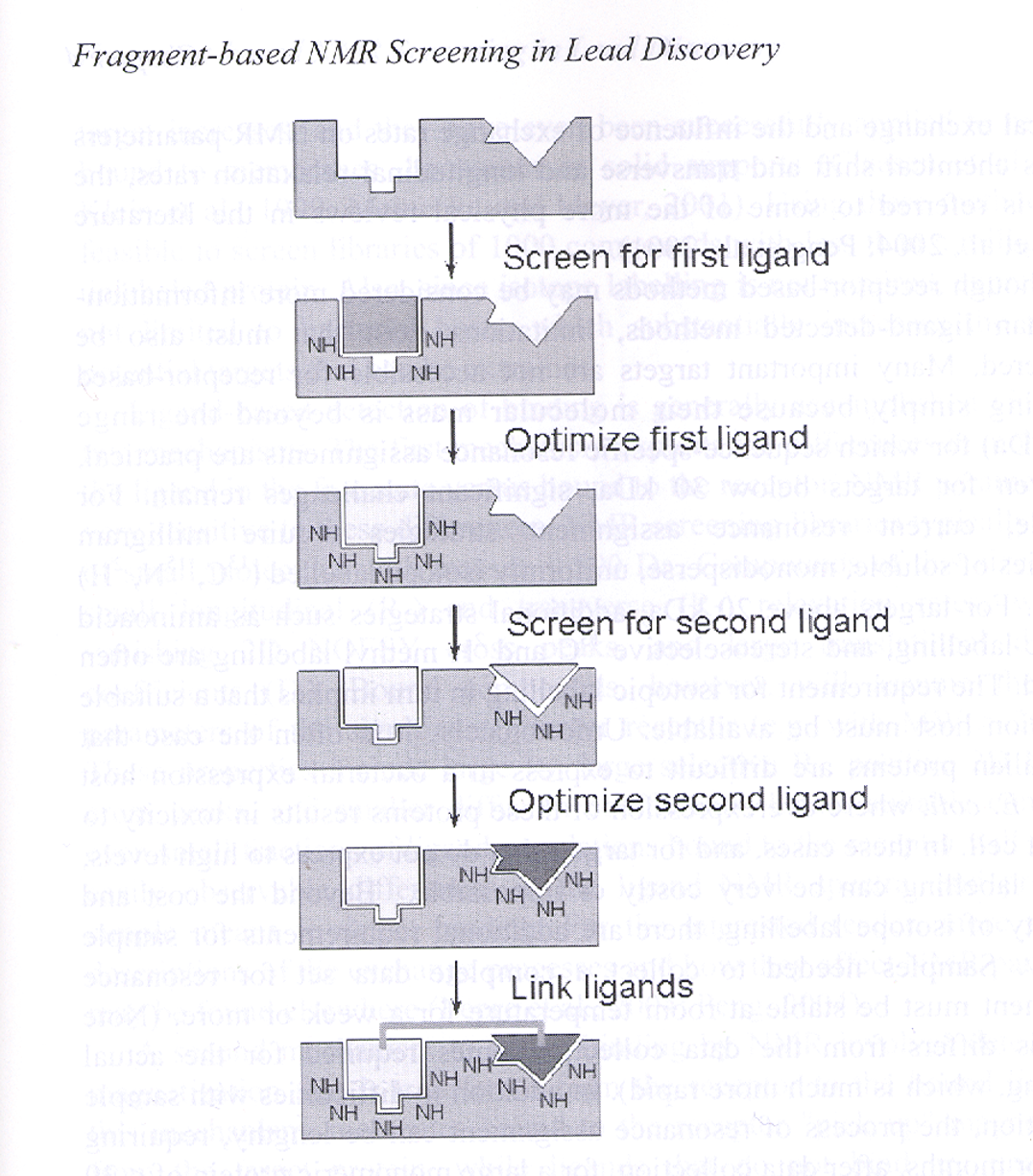 Figure 12. Schematic drawing of the concept of Fragment-Based NMR screening developed at Abbott Laboratories, nicknamed ‘SAR by NMR’ by Dr. Steve Fesik and colleagues. Fragments bound are identified by shifts in the NMR spectra at two proximal sites in the protein target, then linked by appropriate chemical linkers to obtain high potency ligands (~ uM). This process was faster than the conventional methods of the time. (The image kindly provided by Dr. Phil Hadjuk from Abbott Laboratories.)
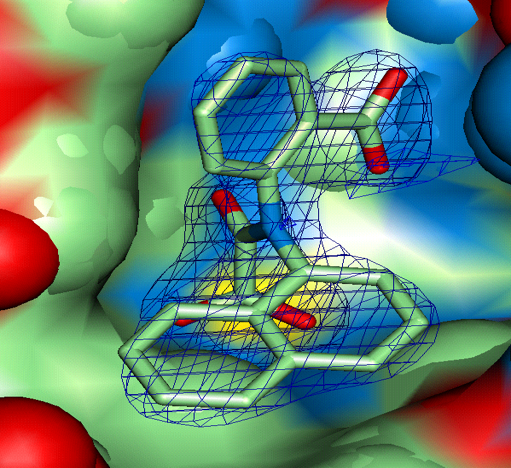
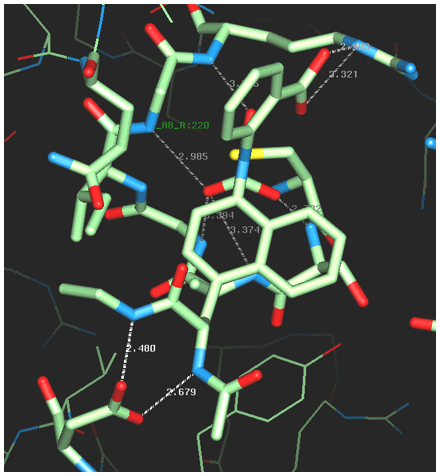
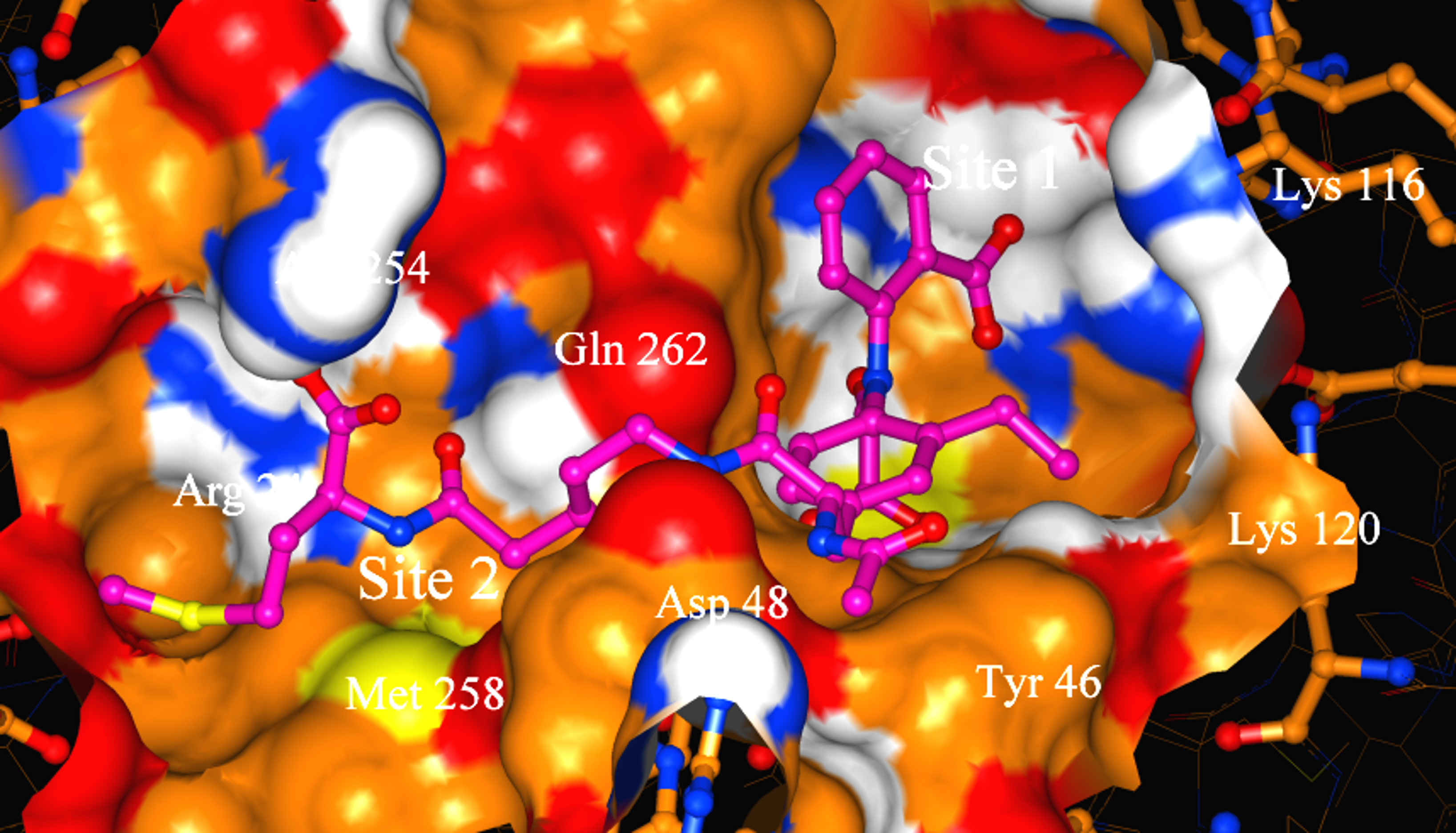
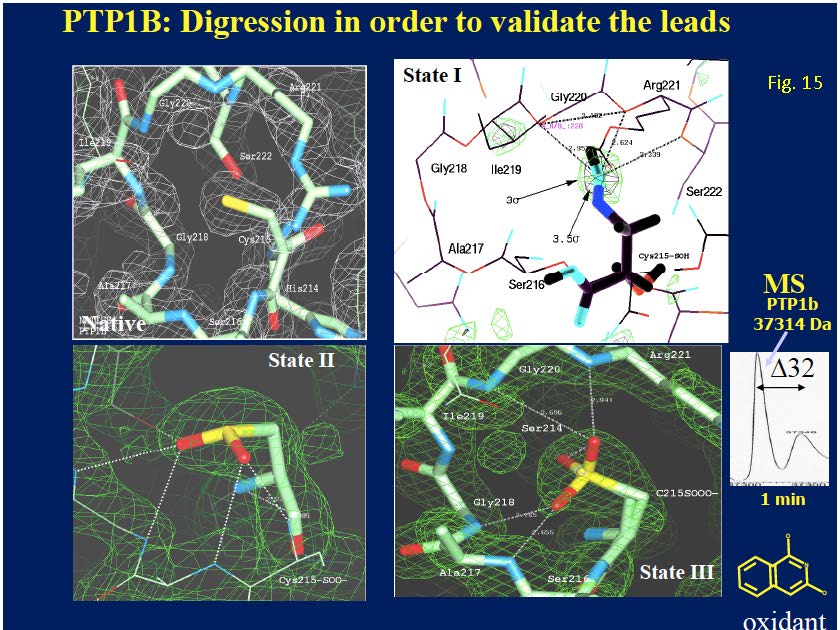
Using isotopically labeled protein, a screening of fragment libraries is performed targeting a known or presumed pocket in the target. After an initial “hit” the SAR of the pocket is explored with related analogs or limited chemistry based on the initial scaffold until an optimal affinity is obtained. Additional pockets or extensions in the proximity of the initial site are explored by screening other libraries again in the presence of the optimized compound. Presumably, a second active compound can be found and optimized. The appropriate linking of the two compounds optimized for the partial cavities should result in a larger compound with a dramatic potency improvement (14). Our relationship with the NMR group at Abbott was cordial but also competitive. Although impressed by the ingenuity of the approach and the concrete result for anticancer therapy, we recognized significant limitations in the power of NMR to distinctly establish the binding mode of the small fragments in larger, more complex proteins. A protein target for which we had excellent crystals diffracting at high resolution in our in-house lab provided an excellent ground for a productive collaboration. Protein tyrosine phosphatase 1B (PTP1B) was a suitable target of dramatic interest in the area of Type II diabetes and related to obesity. HTS (high throughput screening) of the Abbott collection against this protein yielded no suitable hits (see below). PTP1B had a manageable size for NMR small fragment screening and with a limited amount of chemistry found a promising, reversible, inhibitor (Ki = 80 uM) with an oxamic acid head. The best compound was soaked into the PTP1B crystals and binding mode was established unambiguously (Fig. 13a). Successive medicinal chemistry guided by various crystal structures optimized the scaffold in the active site of PTP1B. Structural studies from other laboratories had established the existence of an additional pocket approximately 20 Å away and the combination of NMR screening and medicinal chemistry yielded a compound that was supposed to expand both sites. The double-site compounds were soaked into the PTP1B crystals and convincingly established the methodology and the approach. Further work was needed to achieve enough specificity vs. the T-cell PTP1B to prevent toxicity (Fig. 13b,c).
Figure 13a,b. Implementation of the Fragment-Based approach in the human target PTP1B, combining the identified compounds by NMR with protein-ligand complexes obtained by X-ray crystallography. a. and b. two fragments found and characterized by crystallography on site no. 1 (active site). Initial and optimized, respectively.
Figure 13c,d. Implementation of the Fragment-Based approach in the human target PTP1B, combining the identified compounds by NMR with protein-ligand complexes obtained by X-ray crystallography. c. The extended, linked, double site (site 1 right, site 2, left) as a distinct ligand on the PTP1B surface. d. Surface rendering of a potent analog. (All images from the author’s private collection.)
Any experienced and savvy medicinal chemist would notice that this family (or class) of compounds does not have favorable pharmaco-kinetic properties due to its large size and rather extensive polarity. A duplication of the strategy (screening-optimization-linking) replaced the oxamic acid head by an isoxazole in the first site, and the polar group on the second site by an elegant salicylic acid resulted in a smaller, less polar compound that was active in cell assays. The results and details of this combination of fragment-based and SBDD optimization have been published in several papers from the Abbott groups. A concise pictorial summary is presented in Figs. 13 and 14. A critical issue for the project was to validate by X-ray methods the early hits found by HTS (see below). Once the early bona fide inhibitors were found by the NMR screens and compounds potent enough to be seen in crystal soaking experiments, the PTP1B team had the technology necessary to optimize the compounds quickly using the two available sites (Fig. 13). Optimization to a more favorable pharmacokinetic profile of the compounds was also achieved rapidly with the knowledge of several protein-ligand complexes (Fig. 14). A summary of this landmark validation of the fragment-based approach to ligand design and optimization can be found in reference 15.
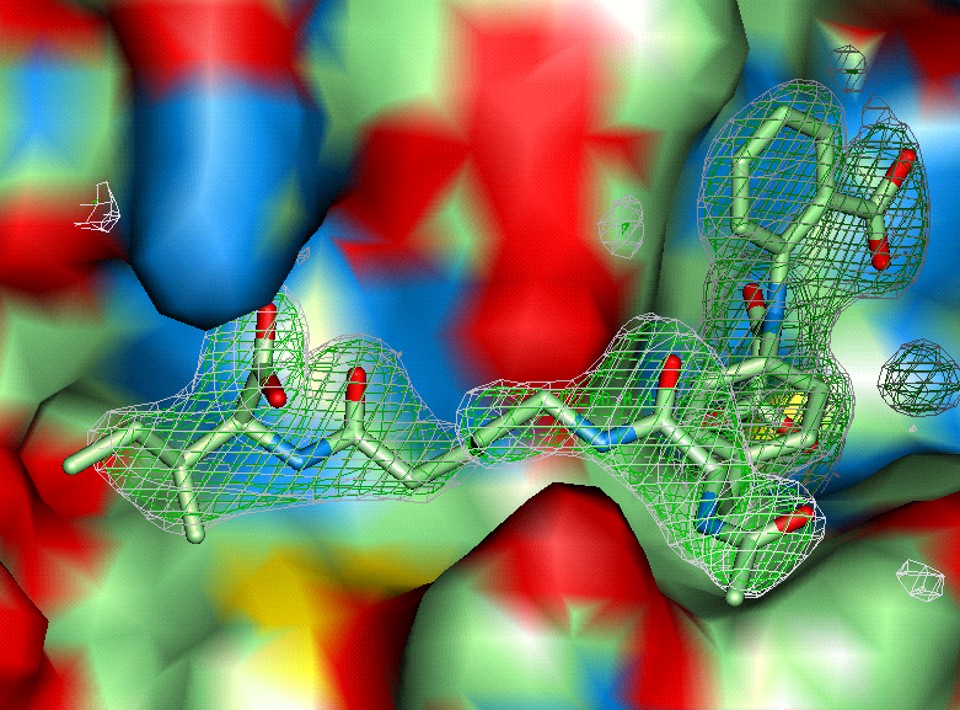
Figure 14. Optimized double site inhibitor of PTP1B. The double site inhibitor had smaller size (lower molecular weight) and much lower polar surface area (PSA) after replacing the oxamic moiety in the active site (right, site 1, Fig. 13d)) by an isoxazole scaffold, and the carboxyl group on the second site (left, Fig. 13d)), with a modified salicylate group. Compound depicted in this figure was active in cells (PDB: 1xio). (Image created from the PDB entry by the author.)
8.5. Subtle insights along the way (protein oxidation). Through my professional years as a protein crystallography in the pharmaceutical industry, I have probably looked at and studied hundreds or even thousands of electron density maps, looking for subtle details of the conformation(s) and interactions of hundreds of small molecule ligands with their corresponding target in crystallographic complexes, either after soaking or via co-crystallization. This was particularly true when the crystallographic screening was fully implemented at Abbott Laboratories. Of course, there were successes and failures. I am going to focus on a particular insight that came to me after months of agonizing work with PTP1B, a critical protein in the pathway of glucose signaling and an important target for the possible treatment of type II diabetes. Like many other pharmaceutical companies, Abbott Labs had a program targeting PTP1B with the goal of developing potent and reversible inhibitors of this target for the treatment of the conditions mentioned above. Following the most successful methodology of the time, HTS (high throughput screens) were performed on the extensive Abbott compound collection in an attempt to identify active compounds that could be used as starting scaffolds for further medicinal chemistry optimization. The most striking result of these screens was the identification of a couple of low molecular weight (~ 200 Daltons) isoquinoline diols that appeared to inhibit PTP1B at the sub micromolecular level (0.6 uM). The project team and the medicinal chemists within the team were particularly interested in seeing how these compounds bound into the active site and how they could be modified to increase their potency. As the protein crystallographer in the team, I was assigned to find the answers to these important questions. Several months of perplexity and frustration followed. I repeatedly soaked fresh crystals of PTP1B with concentrated solution of the isoquinoline diols and collected data of excellent quality in our in-house laboratory. The compounds could not be found near the active site or anywhere else within the pockets or crevices of the protein. Eventually, as I became more familiar with the detailed density near the active site, what appeared to be minor alterations of the native density became consistently apparent: something was going on! I consulted with my chemist/biochemist colleagues at Abbott and eventually a logical explanation was found. Somehow, the small compounds were oxidizing the active site cysteine (Cys215) in various stages. Controlling the soaking time, concentration of the ligands in the soaking solution and the age of the crystals, I was able to characterize the three stages of the oxidation of the –SH group in Cys215: sulfenic (-SO), sulfinic (-SOO) and sulfonic (-SOOO). The compounds inhibited the enzyme by oxidating the active site Cys215 and rendering it inactive (Fig. 15). This subtle chemical effect prevented the binding of any other ligand to the active site by changing dramatically its chemical properties. The successful soaking of ligands in native PTP1B crystals required a careful control of the oxidation state of the protein and crystals well beyond the standard addition of DTT and other protective additives.
Figure 15. Characterization of small covalent inhibitors of PTP1B by X-ray crystallography. The native (unperturbed) active site of the enzyme is shown in the upper left panel. A small isoquinoline diol (lower right) was found to inhibit PTP1B at the micromolecular level (0.6 uM)l. Attempts to obtain the compound mode of binding to the PTP1B failed repeatedly until it was realized that the compound was oxidizing the active site Cys215 in three different stages: Sulfenic (upper right), (S-O), Sulfinic (lower left) (SO2) and sulfonic (lower right (SO3). Inset shows the shift (+32 Da) in the mass spectroscopy spectrum of the incubation mixture 1 min after incubation. (Author’s personal collection.)
This insight made me realize two things that were important for future projects in SBDD in our laboratory. First, the subtlety of the effects made oxidation very difficult to detect unless you knew exactly what to look for. Second, the power of crystallography to unambiguously explain structurally the mechanism of inhibition of these (and other) elusive compounds. Since many drug discovery projects now rely on X-ray crystallography to validate the results of various types of screening, protein crystallographers can play a critical role in triaging and confirming the mode of action of putative inhibitors. I should add that since then, I have discovered this effect in various structures deposited in the PDB involving cysteine residues, making some of the claims made in the published articles rather questionable. After these extensive collaborations with the NMR group within Abbott Laboratories and other pharmaceutical laboratories the combined role of protein crystallography and NMR in drug discovery was established (Fig. 16)and it is widely recognized in the field (16).
Figure 16. Synergistic application of protein crystallography (top) and NMR spectroscopy (lower) in the drug discovery pipeline. This summarizes the complementary methodologies used at Abbott Laboratories to expedite structure-based drug design early in the 21st century.
8.6. Humidity control for ligand soaking. As a protein crystallographer in the pharmaceutical industry, many of my experiments consisted in soaking a crystal of a concentrated solution of a compound (or a mixture of compounds when using limited cocktails, typically in DMSO). Through the years, I have experienced the frustration of damaging the diffracting quality of hundreds (if not thousands) of beautiful crystals by this procedure. Quite often, the procedure results in damaged crystals that barely diffract. It was only after my retirement from Abbott Labs in 2008 that I had the opportunity to address this problem, using an instrument and approach that used a critical property of macromolecular crystals: their diffraction quality depends on keeping them in an optimal moist environment. This observation goes back to the publication of Bernal and Crowfoot on the successful diffraction obtained from pepsin crystals mounted in a sealed quartz capillary. Another engineering innovation from a structural group in Germany (Rieffesaker et al.) had made possible the mounting of native protein crystals without having them enclosed in a sealed capillary (“free-mounting system”). By combining miniature jet streams of dry and moist air near the crystal it was possible to control the relative humidity of the crystal being exposed to the X-rays. This device had been used to optimize the diffraction quality of mediocre or even poor-quality crystals to enhance their diffraction properties. The availability of this instrument was a revelation to me, since I found that this was the ideal device to test the notion that native crystals, damaged by a soaking protocol with ligands, could be recovered and analyzed for the binding of small molecule ligands. With the collaboration of Dr. A. Guasch, J. Pous and I. Fita at the Parc Cientific Barcelona, we were able to prove that this was indeed possible for certain protein crystals (PurE from B. Anthracis) (17). Whether this observation can be extended to other macromolecular crystals of other valuable pharmaceutical targets is still an open question and remains unexplored.
8.7. The personal impact of what we do. I would end this section with a note regarding the impact that protein crystallography, structure-based drug design and drug discovery does have can have at a personal level. Just by the contingency and fragility of life, early in the spring of 2015, our older grandson (Mateo, aged 5) was diagnosed with ALL (acute lymphoblastic leukemia). The news was devastating for the entire family. Yet, after reading and learning more about the newly available treatments developed in the last decade, the prognosis had changed dramatically. Because of the efforts of dedicated biomedical workers in hospital and clinics around the world, including medicinal chemists, pharmacologists and protein crystallographers in academia and the pharmaceutical industry, the prognosis for a full cure ranged between 80-90% — what a dramatic change. I could have never imagined that the early research of J. Kraut and collaborators characterizing the binding of methotrexate to DHFR (dihydrofolate reductase) could have any relevance to the therapeutic agents used to treat our grandson. After months of grueling treatment, requiring long hospital stays and periodic visits, he is doing well (as of this writing he is currently a high-school sophomore) and we all look forward to the time when these difficult and trying months will be a distant nightmare in our lives. I do mention all these personal notes to emphasize that what we do does matter. Our dedicated work, even if not devoted to any particularly successful cure in the clinic, is complemented by the same devoted work by thousands of other workers in the biomedical fields where breakthroughs are accomplished and lives saved. I would like to end this section with an additional sentence (in the words of Michael Rossmann, one of our pioneers and most prominent and dedicated structural biologists of our times: “There are two reasons why we do research. One is the personal enjoyment of discovery, and the other is the desire to contribute something to the benefit of mankind.” I would like to add a third one: “The fulfillment of our full potential, for our personal satisfaction and to fully contribute to the benefit of society.” I would like this to be the message that I also wish to pass along to the future generations of protein crystallographers and structural biologists.
9. Drug discovery beyond Abbott Laboratories 9.1 Atlas of Chemico-Biological Space The praises and achievements of SBDD during the approximately thirty years (1985-2015) that has been in the spotlight cannot be denied. From the early reviews after the first decade, to the one by Blundell and colleagues approximately at the mid-point (18), the consensus is that its accomplishments are superior to its limitations. I do share that view also. However, from the perspective of other design and optimization endeavors in human technology, I feel that we are still far away from having reached an efficient path. In the last decade, there has been an undercurrent of criticism and self-evaluation as to the “lack of productivity” in the pharmaceutical industry. Naturally, there is the issue of the complexity of the biomedical sciences, individual responses to drugs, federal regulations and other questions that seem to be inherent to the difficulties of finding, designing and approving new drugs in the clinic. Possibly my background in the physical sciences is tainting my judgement and making me imagine things that are not possible in the future of drug discovery. Nonetheless, I would like to present a few ideas for the future generations to consider. The extensive use of SBDD and the predominant use of the potency of the compounds (measured as Ki, IC50, Kd or related variables) often resulted in compounds that were potent but did not have the right physico-chemical properties to have favorable pharmacokinetic properties — typically very large and very polar. In the late 1990s, the guidelines suggested by Lipinski and known as the “rule of five” (Ro5), provided an easy rule-of-thumb to address some of these issues and has been very extensively (and many would argue indiscriminately) used in the drug discovery community as an effective way to weed out the unfavorable compounds along the discovery path. I was indeed exposed to these principles during the later years at Abbott Laboratories but on the tenth anniversary of the publication of Lipinski’s paper, I wrote a brief essay arguing that we should look for other variables to guide drug discovery (19). Variables that could combine the potency and the corresponding physicochemical properties in a concise way and that could be used to monitor the optimization process without the use of the hard boundaries suggested by the Ro5. In my discussions and seminars, I used the example of the concept of density. Density (=m/V), as the combination of mass (m) divided by volume (V). This simple combined variable is a very important physicochemical property that is essential to characterize many physical phenomena, and that is superior to the two variables that define it (mass and volume) used separately. In the last years of my stay at Abbott Laboratories and in collaboration with Jim Metz, we extended the notion of “Ligand Efficiency” that had been originally proposed by Hopkins and colleagues (2004) to characterize the quality of molecular fragments (20). We suggested that a combination of two efficiency variables (BEI: size efficiency and SEI: polar surface efficiency; ‘Ligand Efficiency Indices’ or LEIs) should be used to guide drug discovery in a more effective way. 9.2. Structure-Based Drug Design in the academic environment: freedom and commitment. My retirement from Abbott (2008), gave me the opportunity to develop and expand the ideas of LEIs in the broader framework of ‘alternative variables’ to guide drug discovery, beyond the time-honored guides of the affinity and specificity towards the target. The initial ideas published in 2005 have been significantly expanded (21, 22) because I do believe that they have merit and could expedite drug discovery and make it more efficient by applying rigorous methods of multi-parameter optimization that are now being used in powerful software applications (23). The combination of ‘Ligand Efficiency Indices’ with the algorithm and approaches of AI, using LEIs as alternative optimization variables, could offer unexpected synergies (24). Time will tell. I do hope to be able to stay scientifically active and alert to see what happens at least for the next decade. The future generations of drug discoverers will have to take the torch. In addition to this project, my academic inclinations made me look for other opportunities to do research in structural biology and drug discovery at the University of Illinois Chicago (UIC), where I was appointed an Adjunct Professor to the Center for Pharmaceutical Biotechnology in 1999, supported by Prof. Michael Johnson. I was able to do interesting research projects, and he gave me the opportunity to develop my ideas on Ligand Efficiency Indices (see above). After my retirement from Abbott, I was also able to develop collaborations with academic colleagues in Spain (Parc Cientific Barcelona, Prof. Ignacio Fita; University of Alcala de Henares, Prof. Federico Gago), and also with groups at the European Bioinformatics Institute, Dr. John Overington, who developed the chemo-biological database ChEMBL. Incorporating the ideas of the AtlasCBS into the chemo-informatics Software StarDropTM, allowed me to collaborate with the company Optibrium in the U.K. Currently, I am affiliated to the Institute of Tuberculosis Research (ITR, Director Prof. S. Franzblau) at UIC where I have been doing SBDD, mainly characterizing the mode of action of potent anti-TB agents derived from natural product screens, namely rufomycin (25) and ecumicin (26). Combining these results with the improved in silico approaches currently available continues to inspire and stimulate my involvement in structure-based drug-discovery.
10. Science/Crystallography and the Arts Much has been written about the connection about science and art, and it is not my intention to scholarly review such a vast area in these biographical notes (see for example ref. 27). Possibly, a future essay will cover this issue in more detail. As a young and curious student, I was attracted to learn about both. My choice of a scientific career prevented me from being a scholar in the humanities but I always had enough knowledge of art through my father to know how to appreciate it and respond to its different manifestations: architecture, sculpture, painting, specially music, and even the art of good cinematography (7th Art) of which my father was very fond. As I matured scientifically and read extensively about the themes of scientific discovery and artistic creation, I began to see them as the two most critically human activities of the human mind. In my mind, nothing could compare to these manifestations of the human psyche and to me they are totally equivalent. A brilliant scientific paper (i.e., Einstein’s theory of relativity) related to the masterpiece of an orchestral composition (i.e., Beethoven’s 5th Symphony). We are lucky that through channels of scientific publication and communication on the one side, and by the institutions of symphonic music on the other, we human beings can enjoy both, although the former might require a bit more dedication and effort than the latter. Probably, most of the readers of these lines would agree with the notions expressed above. However, what about the relationship between the sciences and the arts, in the operative sense. Do they interact and influence each other? In the Western thought, much has been written about C.P. Snow’s two cultures and the gap between the two. As I continued to grow in my scientific profession and persisted in feeding my artistic curiosity, the barriers began to crumble and I am now of the opinion that there has always been a close interaction between the sciences and arts and I will discuss with some detail a crucial connection that it is very relevant to X rays and crystallography. My excursion into the domain of playwriting opened a window of enquiry that revealed something totally unexpected. I began sketching and writing a play about the historical encounter of John D. Bernal (one of the most charismatic pioneer crystallographers of England) and Pablo Ruiz Picasso, probably the most influential artist of the 20th century, if not of all times. They were both known for their left-wing politics. Bernal was a devoted supporter of the Soviet Union and declared member of the Communist Party. Picasso had a more ambiguous political affiliation, although at one time he was also a member of the Communist Party. They met at Bernal’s flat in November 1950, while attending a pacifist meeting that was later suspended by the British government. The encounter has been documented in the biography of Bernal by A. Brown (28). There are not many details of the conversation that took place between the two on that momentous evening, but Picasso left a mural drawing at Bernal’s apartment that is now at the headquarters of the Wellcome Trust in England (Fig.17).
Figure 17. The drawing that Picasso left in the flat of John D. Bernal in 1950, when he visited England for a peace congress at Sheffield. The mural is now at the headquarters of the Wellcome Trust in London (28).
A dramatic rendering of this encounter was offered in the play Bernal’s Picasso, which I wrote in collaboration with Jill Campbell. It was presented as a staged reading at the 2008 APS USERS meeting (https://caz.crystaledges.org/drama/). Although it was a step in the right direction, I think that further work on this script is needed and could be an intriguing way to present the interplay between science and art, centered around crystallography (Fig. 18). Picasso abhorred symmetry and certainly did not consider it Art, even in the Graphic of M. C. Escher. However, through the play, Picasso realizes that the unique objects buried in the asymmetric unit of protein crystals could be considered Art at the atomic level. Our mathematical hero, J.B.J. Fourier, also appears in the play and Rosalind Franklin explains to him how the mathematical series that he invented to solve the equations of the heat transmission in his memoir of 1822 were used to ‘draw’ (sketch) in different detail the forms of those ‘atomic masterpieces’.
Figure 18. Bernal’s Picasso. A snapshot of the stage reading of the play at the user’s Meeting of the APS on Sunday, May 4, 2008. The characters of the play are: (front row, left to right) Picasso, J.B.J. Fourier (dressed in costume of the 1820’s in France), Rosalind E. Franklin, Sir William Lawrence Bragg. In the back, Science (left) and Art (right), representing the characters of the mural drawn by Picasso at Bernal’s flat.
Doing as much research as I possibly could around the two characters and the connection between the sciences and the arts, I encountered the scholarly work of the art historian Linda D. Henderson, professor at the University of Texas at Austin. This was a revelation. She had studied and documented a certain and previously underestimated and misinterpreted connection between the discovery of X rays by W. Roentgen in 1895 and the work of Kupka, Duchamp and the cubist paintings of Picasso and Braque among others (29, 30). Any reader interested in this discovery can go to the original publications to fill in the details. I will briefly summarize her findings to document here that the discovery of X rays, a strictly scientific discovery, had a tremendous influence on the artists – predominantly painters – of the cubist and related schools. Artists and the public imagination were fascinated by the notions of additional dimensions of space and time, suggested by the scientific publications related to the theory of relativity. Art historians have written extensively on the impact of these scientific theories in art (31) and Prof. L.D. Henderson critically analyzed these ideas early in the graduate work and also extensively later (32). As a crystallographer, what I found absorbing was her argument about the direct influence of the discovery of X rays and the search for “other views” of reality by the painters in the very early 1900s and particularly the cubists Braque, Picasso and others. The dramatic photographs published by Roentgen in his scientific communications opened the eye of scientists, artists and laypersons alike to an unseen perspective of reality that was beyond the perception of the naked eye. As documented by the work of Prof. Henderson, this notion appeared in the press, popular publications, hobbyist magazines and made it to “artistic manifestos” of the upcoming artistic movements. The discoveries related to radioactivity, invisible particles and rays added to this notion and the imagination of painters and many other artists (i.e., Marcel Duchamp) creating work after work influenced by scientific discoveries. This brief memoir of my wanderings between the sciences and the arts does not allow me to fully expand on this very important concept. I will just quote a brief excerpt from Prof. Henderson’s work and refer the reader to her earlier publication in the Art Journal entitled X rays and the Quest for Invisible Reality in the Art of Kupka, Duchamp and the Cubists (29), as well as her extensive publications on the subject. I don’t think that scientists in general and crystallographers in particular realized the effect that X-rays had on the artists and popular culture of the time. A significant summary document is in the article mentioned above, with remarkable and striking images to that effect. I will just quote a brief popular rhyme of the time that sets the tone for the popularity of the early X-ray photographs and certainly fired up many imaginations among contemporary artists. (Attributed to Charles Kingsley, https://libquotes.com/charles-kingsley/quote/lbt2f9m.) The Roentgen Rays,
The Roentgen Rays What is this craze,
The town's ablaze,
With the new phase of X-rays ways I'm full of daze,
Shock and amaze,
For nowadays, I hear they'll gaze,
Thro' cloak and gown - and even stays,
These naughty, naughty Roentgen Rays.
One of the discoveries of my conscious pursuit of science as a profession and the assiduous interest in the arts is that the two are inextricably linked. Naturally, the scholarly connection discussed above, resulting from a professional and exhaustive scholarly investigation, is particularly reassuring. 11. Notes of a Protein Crystallographer Even as a teenager, I remember having always some kind of notebook where I would put down reflections, ideas, and insights, including trivial things such as movies I had seen and liked. I do not remember any profound ideas making it to those lines but somehow, I found the exercise stimulating and useful. It was as if I had some “quiet time” to talk to myself and reflect upon in the incidents of the day or, more importantly, the future path to follow personally and professionally. I particularly felt the need for those times of reflections the years immediately following my undergraduate degree. I was unsatisfied with the options that were available to me and wanted to find a way to develop my intellectual potential. I found the scientific and intellectual atmosphere of Spain under Franco (1939-1975) particularly oppressive, with the presence and power of the Catholic church dominating many aspects of the educational system in Spain. In addition, most of the professors provided a very narrow and limited view of their fields without inspiring the students with any sense of what the scientific endeavor was all about. The teachings were typically a repetition of the well-established patterns and scientific facts. The inter-relation among the different sciences was never discussed with physicists looking down on chemists, chemists looking down on biologists, and down the scale to the students in the humanities (Faculty of Philosophy and Letters, as it was called in those years in Spain). Certainly, there was not even the remotest notion of any connection between the sciences and the arts. During my years at Abbott Laboratories, daily immersed in the practical applications of macromolecular crystallography, I began writing these brief notes with the intention of making them a regular contribution in the technical journals of our field. I wanted to “soften” them up; to present a wider perspective of the scientific findings. No editor asked me to write a regular column, but I think that I succeeded to some extent in expanding the horizons of the readers of Structure and Acta Crystallographica, predominantly, with brief essays highlighting some historical, human, or artistic connections of crystallography with other branches of science and art. In addition, these brief essays allowed me to understand, document, and establish logical links between the sciences and the arts that I never thought existed before. These ruminations made my intellectual life far richer. Shorter versions of those essays and other ones that were not suitable for publication in scientific journals have appeared semi-regularly in the ACA RefleXions Newsletter, thanks to the courtesy of the editors. I do hope that they have also enriched the lives of the readers. There is one in particular that I feel is quite appropriate to end this memoir, connecting the beautiful rose windows of the gothic cathedrals (Fig. 19) to the mathematical symmetry that they exhibit and the latest results of the structure of the biological structures known as vaults. I do hope that I can still contribute a few more and that they are not published posthumously.
Figure 19. Cover of Acta Cryst Section D. 70(3), 2014. The cyclic symmetry in the rose window on the south entrance (Puerta del Sarmental) of the Burgos Cathedral in Northern Spain, which exhibits a rare 20-fold pattern. The symmetry in the windows is related to the symmetry in biological macromolecules. The essay was one of the ones published in Acta Crystallographica under the series Notes of a Protein Crystallographer. Abbreviated versions of these essays have been published in the Newsletter of the ACA.
I wish to add one more example of the art derived from our three-dimensional structures of proteins. This is exemplified by the sculpture Angel of the West, crafted by Julian Voss-Andreae from the structure of the immunoglobulin domains. The sculpture is on the campus of Scripps Research Institute in Jupiter, FL. The protein structure is embedded within the circumference, referencing Leonardo da Vinci’s Vitruvian Man; it highlights the similar proportions of the antibody molecule and the human body. He has also created a striking sculpture of the collagen structure that is now standing in the campus of Rutger University in New Jersey at the entrance of the building housing the Protein Data Bank.
Fig. 20. The sculptor and his creation. Julian Voss-Andreae and his rendering of the immunoglobin molecule in stainless steel, with the title “Angel of the West”.
12. Reflections As I wrote these notes, it seemed to me that besides the historical facts and anecdotes, a few key points could be relevant to other people’s lives and could help them in their decision and career choices. Contingency of Life and Choices. We can plan our lives in a certain path and direction, but there is always an element of the unexpected and unplanned that should be considered. It is very important to recognize that unexpected option or opportunity, because our minds cannot plan what they do not know. Consider the unforeseen, since it can change your life plans to unforeseen riches. The Transforming Power of Education. I was indeed transformed by the education that I received first in Spain, later in the graduate studies at the University of Texas at Austin, and my postdoctoral years at Purdue University. But in my experience and perception, there was a critical difference between the education I experienced in Spain and the one I received in the USA. The spirit of many universities in Spain at the time was condensed in the words inscribed at the entrance of some of them: Sapientia Aedificavit sibi Domun (Wisdom built its house here), implying that knowledge had its house in those buildings and should not go beyond those quarters. I have seen recently engraved on the stones of the main mall at the University of Texas at Austin its mission: Transforming lives for the benefit of society. In retrospect, I do think that this second calling and vision is more valuable to help the younger generations to realize their full potential and enrich the future of humankind. Instruments vs. Ideas and Results. In discussing advances and progress in our field, we tend to emphasize results and the discovery of novel structures or biological effects. However, advances in many scientific fields depend heavily on the ingenuity and efforts of researchers devoted to design, develop and improve instruments. Certainly, our field of research has depended heavily on the labors on many of those individuals, from the early inventors of X-ray sealed tubes to the enormous number of people involved in synchrotron design, construction and maintenance, to the researchers devoted to build and maintain the equipment in our amazing synchrotron beamlines of the 20th century, and to the instrumentalists now working the development of the X-ray free electron lasers. Our instrumentalist colleagues need to be recognized and acknowledged in their own right for their ingenuity, creativity and hard work. What we do matters! Given my academic interests, I always had the impression that our work at Abbott Laboratories for over 22 years was, in a way, an abstraction – something detached from the realities of everyday life. There were discussions of medical conditions, unmet medical needs, protein targets, clinical candidates, etc. However, the events of the year 2015 related to our grandson’s health were like a tsunami. After the shock, it made me immediately realize that our work and dedication are important, and although it may not appear to be directly connected to human needs, what we do does matter. Our efforts, directly or indirectly, do impact human lives. Therefore, we should be inspired by the words of those that preceded us to continue the research path in whatever endeavor we may choose: “There are two reasons why we do research: One is the personal enjoyment of discovery. The other is wanting to contribute something to humanity and to human knowledge” (M. Rossmann). And not to be forgotten: Third: “Fulfill and realize our human potential, for our own self-satisfaction and to maximize our contributions to society”.
An unexpected honor A brief note should be added here to express my wholehearted gratitude to the ACA as a professional organization that has certainly allowed me to grow professionally from my student days and, indeed, realize my full personal and professional potential, beyond what I could ever had imagined. I am humbled by the honor that was bestowed upon me of being named an ACA Fellow in 2024. 13. Crystal Ball As a young man, I was never happier than when I was studying, reading or writing. Thus, the thought comes to me: what would I do if I were to be a student again?On the biology side I would focus on the vast field of neurobiology. On the structural side, I would explore and study the formation and evolution of dissipative structures discussed extensively in many of Ilya Prigogine’s publications and for which was awarded the Nobel Prize in 1977 (33). Undoubtedly, understanding the molecular and physico-chemical mechanisms of the workings of the human brain is the next frontier in biology and medicinal sciences. I can mention, to whet your appetite, the recent discovery reported in Nature Neuroscience that the “connectome” (the set of brain connections upon a triggering event) is a unique pattern of the individual. As much as we have learned from the various areas of biological and medical sciences, the complexity and subtlety of the human brain is beyond dispute, the leading edge of the biological and medical sciences. The vast areas of neurobiology and brain research appear to me as the next extraordinary challenge. When I think about the study of the physico-chemical phenomena that drive our brain, I always think about a striking metaphor that was mentioned in the introduction to a book by Albert Szent-Gyorgyi (1893-1988). Szent-Gyorgyi was the charismatic and rebellious Hungarian pioneer biochemist who received the Nobel Prize in Physiology or Medicine in 1937 for his work on vitamin C. As far as I remember, I read Introduction to a Submolecular Biology when I was beginning to explore the connection between physics and biology in my undergraduate years in Spain. He graphically presented the following argument. If you give dynamo to a molecular biologist, he will break it down into pieces and explain them in detail, trying to explain how it works. However, if you were to point out to the savvy molecular biology scientist that the dynamo works because of changes in magnetic flux inside the dynamo, he would probably respond indignantly, “That is vitalism!” Another reflection that comes to me when I think about the study of the brain using the methods and concepts that we now call molecular and structural biology is the following. I have written about this in a 2007 ‘Notes’ essay in ACA RefleXions Newsletter related to the future of molecular biology (Quo vadis Structural Biology?) and also in my modest scientific journey (1). I encourage the interested reader to consult these references. To end these lines, I will briefly give the punch line, From our early ages, we have been taught to respond in a very clear and unambiguous way to the question: What are the states of matter? And the answer is, obviously: solid, liquid, and gas, right? Is this true? Is that all? Aren’t we forgetting something? Where do we place the living organisms? From the microscopic bacteria to the magnificent elephants and lions of Africa or the spectacular whales in the sea, without forgetting ourselves, human beings. This simple question and the qualified answer highlight for me that the future study of biological processes and the human brain has to expand the explanatory framework to additional concepts besides the molecular components. Not in the sense of a vitalistic explanation but in the sense of including the concepts and ideas related to “dissipative structures” and non-equilibrium physico-chemical phenomena (see the writings of Ilya Prigogine). Our beloved macromolecular structures represent the parts of this magnificent machinery that make life possible, but we should not ignore the fluxes (nutrients, ions, chemical, electric, and nervous signals, information, etc.) that make those processes possible. It is at the interface, intersection, and interplay of these two poles that I would like to understand the workings of the human brain. Perhaps the expanded notions of systems biology combined with AI and “dissipative structures” could explain in the future, the workings of the human brain itself using concepts developed inside the human brain itself and related to the physics and chemistry that the brain itself has discovered and invented in the last few hundred years. Wouldn’t that be amazing? And since this brief autobiographical memoir discusses the themes of science and the arts, I would like to conclude with an artistic metaphor to illustrate the points made above. Consider a live opera performance. The sheer complexity and beauty of it all. As somebody might say, opera is life itself! Attempts to break it down into parts can identify and separate some clear components: staging, props, singers, orchestra, instruments, and musicians, among many others. Within the stage, there would be structures and components that can be readily identified, including the human beings embedded in the performance. And yet, what drives the opera? The plot and the music: words and notes. Extremely subtle sounds: without them, the other elements would be lifeless. I always think of opera as a “dissipative structure” driven by the music and the words that would disappear without the flow of those two components, no matter how superb the staging might be. Can the parts only explain the opera? Are words alone enough to explain the emotions expressed by characters? Is the music alone enough to drive the action? And in the dilemma posed by the Strauss opera Capriccio: are words more important than music? Or is it the other way around? I leave these questions for the reader and the future generations to ponder as we dedicatedly strive to an understanding of organisms in strictly molecular and physico-chemical terms. Acknowledgement The author greatly appreciates the assistance and dedication of Dr. Virginia Pett, ACA Historian, in reviewing and copy-editing the final version of the manuscript.
References 1. For brevity only a few key references are included in this narrative. Original references in domains outside science such as the section on Science and the Arts have been included. Further technical issues of the themes described can be found in the World Wide Web. Abad-Zapatero, C. Crystals and Life: A Personal Journey; Intl Univ Line, 2002. 2. Abad-Zapatero, C. “Celebrating Macromolecular Crystallography,” Arbor, 2015,191: 772. 3. Harrison, S.C., Olson, A.J., Schutt, C.E., Winkler, F. K., Bricogne, G. “Tomato Bushy Stunt Virus at 2.9 Å resolution”. Nature 1978, 276:368-373. 4. Abad-Zapatero, C., Abdel-Meguid S.S., Johnson J.E., Leslie, A.G.W., Rayment, I., Rossmann, M.G., Suck, D., Tsukihara, T., “Structure of Southern Bean Mosaic Virus at 2.8 Å resolution. Nature 1980, 286:33-39. 5. Liljas A. et al. “Structure of Satellite Tobacco Necrosis Virus”. J. Mol. Biology 1982, 195(1):93-108. 6. De Chadarevian, S. Designs for Life. Molecular Biology after World War II. 2002. Paperback Edition 2011. Cambridge University Press. 7. Jones, T.A. “A graphics model building and refinement system for macromolecules.” J. Applied Crystallography 1978, 11:268. 8. Abad-Zapatero, C. Griffith, J.P., Sussman, J.L., Rossmann, M. G. “Refined crystal structure of dogfish M4 apo-lactate dehydrogenase”. J. Mol. Biology 1987, 198:445-467. 9. Bolin, J.T., Filman, D.J., Mathews, D.A., Hamlin, R.C., Kraut, J. “Crystal structures of Escherichia coli and Lactobacillus casei dihydrofolate reductase refined at 1.7 Å resolution”. I. General features and binding of methotrexate. J. Biological Chemistry. 257:13650-13662. 10. Erickson, J., Neidhart D.J., Van Drie, J. et al. “Design, activity and 2.8 Å crystal structure of a C2 symmetric inhibitor complexed with HIV-1 protease”. Science 1990, 249:527-533. 11. Abad-Zapatero, C. “Notes of a Protein Crystallographer: Our Unsung heroes”. Structure 2004, 11:523-527. 12. Bussiere D.E., Muchmore S.W., Dealwis C.G., Schluckebier G., Nienaber, V.L., Edalji R.P., Walter K.A., Ladror U.S., Holzman T.F., Abad-Zapatero, C. “Crystal structure of ErmC’, an rRNA methyltransferase which mediates antibiotic resistance in bacteria”. Biochemistry, 1998 May 19; 37,20: 7103-12. doi: 10.1021/bi973113c. PMID: 9585521. 13. Nienaber, V.L. “Discovering novel ligands for macromolecules using X-ray crystallographic screening”. Nature Biotechnology. 18: 1105-1108. 14. Shuker, S.B. et al. Science 1996 Nov 29;274(5292):1531-4. doi: 10.1126/science.274.5292.1531. 15. Liu, G., Xin, Z., Pei, Z., Hajduk, PJ., Abad-Zapatero, C., Hutchins, C.W., Zhao, H., Lubben, T.H., Ballaron, S.J., Haasch, D.L., Kaszubska, W., Rondinone, C.M., Trevillyan, J.M., Jirousek, M.R. “Fragment screening and assembly: a highly efficient approach to a selective and cell active protein tyrosine phosphatase 1B inhibitor”.J Med Chem. 2003 Sep 25;46(20):4232-5. doi:10.1021/jm034122o. PMID: 13678400. 16. Abad-Zapatero C., Stamper, G.F. and Stoll, V. S. “Synergistic Use of Protein Crystallography and Solution-phase NMR spectroscopy in Structure-Based Drug Design”. In Fragment-based Approaches in Drug Discovery. Edited by W. Jahnke and D.A. Erlanson. 2006. Wiley-VCH Verlag GmbH& co. KgaA, Weinheim. 17. Abad-Zapatero C., Oliete, R., Rodriguez-Puente, S., Pous, J. et al. Acta Cryst. Section F. 2011. F67, 1300-1308. 18. Congreve, M., Murray, C. W., Blundel, T. L. 2005. “Structural biology and drug discovery”. Drug Discovery Today 10, 895-907. 19. Abad-Zapatero, C. “A sorcerer’s apprentice and The Rule of Five: from rule-of-thumb to commandment and beyond”. 2007. Drug Discovery Today 12(23-24):995-7. 20. Abad-Zapatero, C., Metz J.T. “Ligand Efficiency indices as guideposts for drug discovery”. Drug Discovery Today. 2005. 464-469. 21. Abad-Zapatero, C. “Ligand Efficiency for Drug Discovery: Towards an Atlas-Guided Paradigm”. 2103. Elsevier-Academic Press. 22. Abad-Zapatero, C. “Ligand efficiency indices for effective drug discovery: a unifying vector formulation”. Expert Opinion Drug Discovery 2021. 763-775, 23. Abad-Zapatero C., Champness, E.J., Segall, M.D. “Alternative variables in drug discovery: promises and challenges”. Future of Medicinal Chemistry. 214. 6(5):577-593. 24. Abad-Zapatero, C. “Artificial intelligence (AI) and alternative variables (AV) in drug discovery: A promising alliance”. Editorial. Drug Discovery Today. 2024. 29(5)1-3. 25. Wolf N.M., Lee, H., Zagal, D., Nam, J.W., Oh, D.C., Lee, H., Suh, J.W., et al. “Structure of the N-terminal domain of ClpC1 in complex with the antituberculosis natural product ecumicin reveals unique binding interactions”. Acta Cryst. 2020. D76(5):458-471. 26. Wolf N.M., Lee, H., Choules, M.P., Pauli, G.F., Phansalkar, R., Anderson, J.R., Gao, W. et al. “High-Resolution Structure of ClpC1-Rufomycin and Ligand Binding Studies Provide a Framework to Design and Optimize Anti-Tuberculosis Leads”. ACS Infectious Diseases. 2019. 5(6):829-840. 27. Art and Science, (2nd edition) Eliane Strosberg, 2015; Abbeville Press Publishers, NY. 28. Brown, A., J. D. Bernal: The Sage of Science. 2005. Oxford University Press. Oxford, UK, New York. 29. Henderson, L.D., “X-rays and the Quest for Invisible Reality in the Art of Kupka, Duchamp and the Cubists”. The Art Journal 47(4), 323-340, 1988. 30. Cubism: The Leonard A. Lauder Collection. Edited by E. Braun and R. Rabinow. The Metropolitan Museum of Art. New York Distributed by Yale University Press, New Haven and London. 2015. Particularly Eric Kandel’s essay on pg. 106, “The cubist challenge to the beholder’s share,” and notes on pg. 309. 31. Kandel, Eric M. The Age of Insight: The Quest to Understand the Unconscious in Art, Mind, and Brain, from Vienna 1900 to the Present. 2012. 32. Henderston, Linda Dalrymple. The Fourth Dimension and Non-Euclidean Geometry in Modern Art. (Revised edition). (2013) MIT Press. Cambridge, Mass and London, England. Particularly Reintroduction on pg. 15. “Augmenting a 1983 History of the Fourth Dimension in Culture and Art (1900-1950)”. 33. Kondepudi, D. K. & Prigogine, I. Modern Thermodynamics. From Heat Engines to Dissipative Structure. Reprinted 2000. Wiley-VCH and John Wiley & Sons. 2000. |