Memoir - Ronald E. Stenkamp (1948 - )
Memoir | Publications | Curriculum Vitae | Videos | Slides | Articles | Obituary
ACA Living History
2019

I attended my first scientific meeting as a junior in high school in August 1964. NASA came to my hometown, Bend, Oregon, to see how the astronauts could walk on lava fields in their lunar space suits. I didn’t get to see Walter Cunningham take his test walks, but I sat through several scientific talks by geologists, debating whether the craters on the moon were of volcanic origin or the result of meteor impacts. Two of us locals sat in the back of the high school auditorium (1500 seats) and watched about 100 participants talk at the meeting. I didn’t understand much of it, but I thought it was boring, and if that’s what scientists did for a living, maybe I’d do something else.
While I was a good student in a broad range of courses, I was already thinking science wasn’t very inviting. In response to Sputnik going up in 1957, science educators had generated all sort of special programs and tools to get kids interested in science, and I ran into that in a serious way in 6th grade. I learned all about hypothesis generation and hypothesis testing, and I was not impressed. I still have trouble with hypothesis-based science. I’m just not oriented to thinking about things that way. Asking questions is what I enjoy doing. Posing hypotheses isn’t.
I did well in school, and in junior high, I started thinking of myself more as a scholar and student. By the time I graduated, I was close to the top of my class as far as grades go, and I’d taken as many honors classes as I could (but not AP biology). Without planning it, I’d received a liberal education (except I’d managed to avoid learning a second language because it conflicted with band and advanced math). I greatly enjoyed mechanical drawing, and I’d out-run Mr. Lively’s curriculum in Advanced Mechanical Drawing. He generated new projects for me, and engineering looked like a possible field for me to pursue when it came time to consider colleges.
Bend is not the most cosmopolitan place in Oregon. In 1966, the nearest four-year college was the University of Oregon (UofO), 125 miles away in Eugene. Oregon State University in Corvallis was just a bit further away than that. And while I considered smaller liberal arts colleges in the Pacific Northwest, Mom and Dad’s finances weren’t going to be enough for that. A substantial local scholarship came through my senior year, so I could go away to college, but which should I choose? I liked the idea of liberal education, and Eugene seemed like a more liberal place than Corvallis, and the school colors (green and gold) matched my grade school colors, so I decided to go to Eugene. (“West of the mountains, and east of the sea, down in the valley’s a good place to be.”)
One attractive program at the UofO was the Honors College (HC) which provides smaller courses focused on a core curriculum in the humanities and liberal arts. I applied to the program and was accepted. It became the focus of my undergraduate life and provided an environment promoting classes with a smaller group of students than normally found at large universities. There was also a special center that provided a place to work with the other HC students, and I made many friends there, including my future wife, Larilyn.
Before joining the HC, I had chosen chemistry as my major, based largely on enjoying my high school chemistry class with Mrs. Cruickshank. When I started talking with other HC students about what we wanted to do with our lives, two of them expressed interest in molecular biology. I’d never heard of that. This was 1966, and Kendrew, Perutz, Watson, Crick, and Wilkens had gotten their Nobel Prizes just four years earlier (1962). (Along with Steinbeck (Literature) and Pauling (Peace)). Molecular Biology was a brand-new field, but the UofO already had an Institute of Molecular Biology organized to bring interdisciplinary studies across departmental lines (along with other institutes). But it was too soon to affect my career choice at that point.
When classes started in the fall of 1966, my school days started with the honors section of freshman chemistry. Many of the students in the class were HC students so we got to know one another well. They seemed sympathetic when I had a lab accident early that quarter that foreshadowed much of my chemical career. One of our first tasks was to produce a dichromate/sulfuric acid cleaning solution for quantitative analysis lab. I made the solution OK, but a few days later, when I lifted the liter bottle up from my cabinet, I didn’t lift it quite enough, and it clipped the stone counter top, about ¼ inch above the bottom of bottle. The bottle broke all the way around, and a liter of cleaning solution hit the benchtop, spilled down the front of the cabinet, and splashed a few drops on my cords. The TA (Lefford Lowden) quickly grabbed the dirty bicarbonate bucket and got the spill under control, but I spent the rest of the lab period cleaning up the mess. The department had to replace the hardware on the cabinet because the acid corroded the lock and hinges. It’s good I wasn’t terribly insightful, or I might have decided to switch fields then.
I had a fair amount of growing-up to do in college. I came from Bend thinking I was a pretty smart guy capable of learning things and getting good grades. It turns out a lot of bright people go off to college, and many of them were a lot brighter and hard-working than I was. It was harder for me to get good grades in college, and this was a big blow to my ego. I grumbled a lot about the lousy teachers in college. Clearly, if they knew how to teach, I would have been doing better academically. Prof. Donald Swinehart was our instructor for freshman chemistry lab, and I talked a lot with him about teaching and learning. He wasn’t very sympathetic. He told me about his experience in college where he decided to master a class in spite of the professor. I thought that was outrageous, maybe even stupid, but several years later, I ended up thanking Swinehart for that. In addition to listening to my complaints, Swinehart was willing to let me take a reading course with him where I could learn about special topics of interest to me.
One chemistry question that I’ve barely started to understand fifty years later concerns chemical bonds. I spent some of my undergraduate years in the library, looking at history of science books and others indicating what science was all about. I was greatly impressed by Pauling’s “The Architecture of Molecules”, and Roger Hayward’s artwork of molecules was fantastic. I didn’t understand what the sticks connecting the balls really meant, but it looked like mechanical drawings of molecules. Maybe looking at molecular structures could combine my science and mechanical drawing interests?
When summer break came, I returned to Bend and got a job unloading lumber from boxcars at a molding mill. My parents contended that that job convinced me that I wanted to keep going with college rather than drop out and go to work. I’m not sure I ever had any doubt about that, but my first year of college had been tough on my self-image.
One feature of the Honors College core classes was that after taking them for three quarters, you had to pass a comprehensive exam to remain in good standing in the HC. However, another option was to “challenge” a class by just taking the comprehensive exam. If you succeeded, you could get credit for the class without actually taking it. The exams were given in the spring and in the fall. People taking the classes usually passed the exam in the spring, but in case they didn’t, they were given another chance in the fall.
The history comprehensive exam was feared by most students who took the course, but I’d always enjoyed reading and thinking about history, so I figured I could challenge the history exam. So, for my summer “fun” project, I did all the readings for the HC history class. That meant I needed to do daily history reading assignments through the summer to cover the material discussed in class during the previous nine months. I borrowed the books and notes from a friend who’d passed the course that year, and I managed to read and study nearly all the assignments that summer. I think I balked at reading St. Thomas Aquinas and maybe St. Augustine. I did well with the fall comprehensive exam, and it promoted my confidence in doing independent study.
My memory of my sophomore year is that it was an emotional disaster. In hindsight, it wasn’t a real disaster like those many deal with, but it was a very challenging year that caused a lot of self-assessment My basic problem that year was organic chemistry. And I was a chemistry major! The first two quarters were about reactions (which I’ve never understood) and NMR (I don’t remember where methyl protons come in the spectra). Third quarter had more physical organic content, so my understanding and grades went up for that. But lab was awful. One of my two worst grades on my transcript was from third quarter organic lab. My lab skills (and luck) were not compatible with qualitative organic. I had one unknown that I could not make derivatives of. Even the prof couldn’t do it, and the unknown was a natural product he’d pulled off his shelf. But in a concession to justice, he gave me half credit. Half credit! I had trouble with that class in addition to the intellectual content. And I’m not at all bitter, 50 years later.
Further complicating my sophomore year were physics and a second year of math. The first quarter was linear algebra. It was interesting that the prof couldn’t do the homework problems. He started the second quarter (differential equations) the same way, so I dropped the class and took it the next year. Overall, by the end of the year, I figured anything had to make the next year better than this one.
After a summer working on a surveying crew (which paid enough to cover half of my school expenses), I returned for my junior year. Physical chemistry was a lot better for me. I could understand much of it, but it wasn’t terribly exciting. By the end of the year, I was thinking I still needed to find some chemical topic that was exciting and inspirational. This was near the height of the Viet Nam war protests, so campus life was exciting. I was also thinking more positively about my academic progress, so while I was still wondering what I’d do for a career, I was confident I’d figure it out, eventually.
Then a wonderful thing happened. Brian Matthews joined the faculty. He joined the Physics Department and the Institute of Molecular Biology, and he was going to do something called protein crystallography. I had decided to stay in Eugene for summer school this year, mainly to take care of my foreign language requirement, take a well-regarded Shakespeare class as well as a geometry class. I also arranged with Brian to take a reading class with him where I could learn about crystallography.
And when school started in the fall, I asked if I could do my senior Honors College thesis with him. He agreed and set me to solving a small molecule structure. Bill Simpson’s lab was interested in organic compounds with metal-like spectroscopic properties, and they wanted to know what the crystal structure was of one of their compounds. Brian’s X-ray lab was just setting up and had an Enraf-Nonius Weissenberg camera, so he had me collect diffraction data for 3-bis(dimethylamino)-trimethinium perchlorate using film packs generated on that camera. There was no film scanner available, so I eye-estimated the intensities on those films. I generated an intensity scale by exposing a single reflection for various lengths of time on a film. This gave a spot with the same shape and extent as those on my data frames. And then I spent several months over a light box determining the relative intensities of the reflections. With a bit of FORTRAN programming, I then scaled the film packs together to generate a data set.
At that point, the school year was finishing, and I had to write my undergraduate thesis. Peter Colman joined the lab as a post-doc, and he ended up solving the structure. Soon after, but after I’d returned to Bend for the summer, a diffractometer arrived, and a higher quality data set was obtained for refinement. My efforts on this structure got me co-authorship with Peter and Brian and started my publication list.
Of course, while this project was important for my future career developments, other important things went on that year. First, Larilyn and I had to make wedding plans. Second, we needed to figure out what to do with our lives. The main thing we were reasonably skilled at was being students. And it seemed the natural consequence of that was to keep going and get our Ph.D. degrees. By that time, we’d pretty much satisfied the requirements for ACS-accredited B.A. degrees. (At the UofO, the B.A. was the accredited degree, consistent with the Honors College’s emphasis on B.A. degrees.) We had the freedom to continue taking chemistry classes, reserving credit for them to apply to graduate degrees if we stayed at Oregon. At the time, the Chemistry Department covered organic chemistry, physical chemistry and biochemistry. So we took three quarters of biochemistry and finally started seeing what molecular biology was about. In addition, we took a fantastic statistical mechanics class (mainly filled with graduate students), and several computer programming classes (assembly language and FORTRAN). Larilyn was a quarter ahead of me in those classes, and I benefitted greatly from that.
But we still needed to decide on graduate school. Larilyn was from Oak Grove (a suburb of Portland), so neither of us wanted to go too far from Oregon. And by then, I knew I wanted to do crystallography. Because of the war, we considered the University of Alberta, and Mike James phoned me to talk about us going north. There was something off-putting though about the parking spots in married student housing being equipped with electrical outlets for engine heaters. You had to figure it must get cold up there. We also considered the University of Arizona, but that was going to be too hot (and they didn’t do protein crystallography then).
And then there was the University of Washington. We considered it and applied. I explained my interest in protein crystallography, and Verner Schomaker, the chair of Chemistry, let us know that protein crystallography was being done in Lyle Jensen’s lab, and interdisciplinary research would be OK with Verner. That all sounded terrific, and in the spring of 1970, Lyle came to Eugene to give a seminar about his group’s refinement of rubredoxin. This was exciting, since it was the first protein to be successfully refined crystallographically. What was more exciting was that after talking with him just before he left for home, he said I should come to Seattle, “and we’ll have some fun.” He had bright blue eyes, and that and his attitude made an impression on me. I was convinced.
So, in late August of 1970, we got married, went to San Francisco for our honeymoon, drove back north to visit with family in Bend and Oak Grove, and moved to Seattle.
Crystallography was been a big deal at the UW. The senior crystallographer was Ed Lingafelter. He’d joined the Chemistry faculty In the late-1930s, straight from being a graduate student at UC Berkeley. He was a physical chemist, and in 1938 or 39, he was joined by his “best” graduate student, Lyle Jensen (one year younger than Ed). Lyle was from Stanwood, a small town about 40 miles north of Seattle. He’d attended Walla Walla College and come to the UW to get his Ph.D. For his thesis, he determined unit cell parameters for a series of long-chain compounds. Once he obtained his Ph.D., Lyle joined the Manhattan Project in Chicago and worked with plutonium compounds. He left that position to teach at a church-sponsored college before going to The Ohio State University to work on the physical chemistry of liquid hydrogen. Soon after that, the UW needed additional instructors to deal with the large number of returning GIs, so he came back west and took up an instructorship in Chemistry. In 1948, just after the UW Medical School opened, he talked with the chair of Anatomy who offered him a junior faculty position the next day. Stan Bennett had a very broad view of “anatomy” and thought crystallography and electron microscopy would eventually be important for studying biological structures. (Stan Bennet was a very wise man.)
The third senior crystallographer was Verner Schomaker. Verner was a Cal Tech product where he did a lot of electron diffraction of compounds of interest to Pauling. There are many footnotes in Pauling’s “The Nature of the Chemical Bond” referring to “V. Schomaker, unpublished results”. The story I heard was that Pauling would get interested in some M-X bond and get Verner to use electron diffraction to obtain the M-X interatomic distance. In the mid-1960s, after years at Cal Tech and Union Carbide, Verner became chair of the Chemistry Department at the UW.

Ed Lingafelter and Verner Schomaker
By 1970, other crystallography faculty at the UW included G.H. Stout in Chemistry, Jon Herriott in Biochemistry, and Art Camerman in Neurology. Subrata Ghose joined Geology sometime in the 70s. There were many graduate students, post-docs and research associates associated with these faculty, so there were enough people solving enough structures to support a weekly X-ray seminar. It was a wonderful environment for learning the fundamentals and cutting-edge techniques in crystallography.
Our first year at the UW was filled with normal graduate school issues. We had coursework to manage, teaching assistantships to master, research groups to join, etc. Larilyn joined Ernest Davidson’s quantum mechanical group, and after a bit of negotiation with a new chair of Chemistry, I could work in Jensen’s group. (Lingafelter was my official advisor, but Lyle was in charge and made any decisions a supervisor had to make. This qualified me as Ling’s “easiest” grad student.)
A guiding principle in Lyle’s group was that small molecule techniques could be used to refine protein models and improve their precision (and hopefully, accuracy). Accordingly, my first projects in the lab were crystal structure determinations of dipeptide structures. The first molecule I tried to solve was a chloromethyl ketone of acetyl-leucyl-phenylalanine. I determined the unit cell and space group using Weissenberg and precession photos, and then we (mainly Larry Sieker) used a Picker FACS-1 (driven by a PDP-8, with paper tape output) to collect a diffraction data set. The structure suffers from super-symmetry, but a bigger problem was that the crystal was greatly radiation damaged before we put it on the diffractometer. I didn’t solve that structure, but it taught me a lot about crystallographic computing.
Following that, I grew crystals of two more dipeptides and solved their structures. I also worked on two small computational projects having to do with less-than reflections and resolution. Crystallography’s appeal for me was (and is) tied up with the idea that solving or refining a structural model is just a big puzzle, and the big question for me has been to see if I understood my craft enough to solve the puzzle. My research efforts have focused on crystallographic techniques, and not so much on the molecules being studied or the biochemical questions being asked.
While the research work was of great importance, equally so were the social interactions in Lyle’s research group and in the crystallographic community at the UW and outside the UW. Lyle’s been recognized by many as a gracious, dignified leader. And those characteristics pertained in day-to-day life in his group. He delegated responsibilities well and made us feel like our projects were ours. He would occasionally come by our offices to see how we were progressing, but almost every day, I went to his office to ask questions and just talk about stuff. I still consider him my “boss”, but he was really a friend.
And the supportive environment carried over with the other members of his group. Larry Sieker was the main crystallizer/data collector and for me, the lowly graduate student, he was the number two person in the group. He knew how to get things done and he was as dedicated to doing good science as Lyle was. That was true of everyone in the group, a dedication to doing good work. While several people came through Lyle’s group as post-docs and visitors, the major post-docs who educated me about crystallography and computing were Keith Watenpaugh, Ellie Adman, and Jonathan Hanson. I can’t express how grateful I am for the things I learned from them and their continued friendship.
I also benefitted from interactions with the other crystallographers on campus through the weekly X-ray seminars. For the presentations of new structures, and there were many of them, there was usually a figure showing the bond lengths and angles for the molecules. What was especially fun was to watch the senior faculty (usually Ling, Verner and Lyle) get interested in comparing the bond lengths and angles to see what the bonding was like. If the presenter was particularly successful, he or she could get the old guys talking and arguing and end up using a substantial portion of the seminar time. I don’t remember ever manipulating my talk to succeed at this, but it happened accidentally enough to make it a career goal.
Ling and Verner also were great influences on how I approach problems, but in addition, they were important for my academic progress. The Chemistry Department expected its graduate students to pass cumulative exams to ensure they had a broad understanding of whichever branch of chemistry they were studying. These exams were given twice each quarter on Saturday mornings. If you could pass four of the first six that you took, you became a PhD candidate (and got a pay raise). Or you could pass five of 12 or six of 18. The faculty alternated in producing questions for the exams, so Verner put in a question for the physical chemistry exam about the structure of diamond. None of us did very well with the question, and he pretty much read me the riot act about it. (At least I remember it as the riot act. He might have been very nice about it, but I was a bit scared of him.) Anyway, since exam-taking is very much a game, we figured we were safe when it came to diamonds and didn’t study the structure. Of course, on the next exam, here came another diamond question. This time there was no riot act, but Verner just shook his head at me when we next passed in the hall. After sufficient time, he seemed to get back to thinking I was OK. I now know a bit more about diamond and have a homework question for my crystallography class where the students are told the space group of diamond, the density of diamonds, and the unit cell dimension. From that information they have to calculate the bond distance for carbon-carbon single bonds. The question is one way I can show my gratitude to Verner.
Jensen’s lab attracted many visitors, especially those interested in crystallographic refinement of macromolecules. Seattle was on the way to Japan and Asia, so we often had people come by for short visits during their travels. One of the visitors I most remember was Alex Rich who came by on his way to a fishing trip in Alaska. As he sat in the lab chatting with us, dressed in his fishing clothes, he suddenly pulled out two wire models of the tRNA structure his lab had just solved. Gold- and silver-plated as well. They were probably too big to be good fishing lures, so he probably had other reasons for carrying them to Alaska.
I tended to be shy and withdrawn when the famous visitors showed up. I found it hard to talk with these people who were my great heroes. I’m especially irritated that I never worked up the courage to spend more time talking with Max Perutz or Dorothy Hodgkin. Lyle had spent a sabbatical in Cambridge, so he knew Max from that time. And Lyle and Dorothy had competed on at least one small molecule structure. When Max and Dorothy came to visit (probably more than once each), they stayed at Lyle’s house. Lyle and his wife, Mildred, held wonderful picnics in their backyard for the visitors and the rest of us. I wish I had an opportunity to re-live those get-togethers. I would work a lot harder at talking with the guests.
I attended my first ACA meeting as a grad student when the ACA met in Berkeley. Larry McCandlish (another Chemistry student) and I took the train to California. All I remember from the meeting was being awestruck when listening to the mathematically literate people like Lynn Ten Eyck and Ed Green. There was no way I could understand or keep up with what they were talking about. The other thing I remember about that trip was the mudslide that delayed our return to Seattle. The tracks were blocked north of Redding with a southbound train on the north side of the slide and our northbound train on the south side. AMTRAK found some school busses and exchanged the passengers between the two trains. And then they provided free dining car service for our 22-hour delayed trip to Seattle.
While completing my small molecule projects and writing them up, Lyle and I considered several protein problems for my thesis research. I made a little progress on two initial molecules (azurin and cytochrome cc’3), but the problem that became my PhD project was hemerythrin. This is an octameric, non-heme iron, oxygen-binding protein with a molecular weight of 108,000, found in a few marine invertebrates. It fit with the Jensen group’s overall interest in redox- and metalloproteins. Joann Sanders Loehr at Portland State University collaborated with Lyle and Larry Sieker and provided protein for a structure determination.
Data collection in Jensen’s group was centered on the FACS-1 diffractometer with a sealed tube X-ray source. Larry oversaw the equipment, and his dedication to keeping the green machine aligned and in good condition, and Lyle’s emphasis on precision, were important reasons I managed to solve the structure. The asymmetric unit for our crystal form contained four subunits from two hemerthrin octamers. The structure was solved at 5 Ångstrom resolution using a single mercury iodide derivative and its anomalous scattering signal. There are about 7500 reflections out to that resolution, and the quick step-scan data collection protocol (developed largely by Jonathan Hanson and Keith Watenpaugh) resulted in our collecting 1500 reflections per day.
At that point, I was becoming a bit desperate about having a thesis project, and then the paper tape messed up, and we lost a large part of our data. There wasn’t time to re-collect it. We still had a printout, so I ended up key-punching about 2000 reflections onto computer cards so I could keep the project going.
Then it was time to compute on the campus’ CDC6400 to generate a difference Patterson map for the derivative. Ellie Adman had solved a multi-site Patterson in working out the structure of ferredoxin, so she’d set an example for working your way through a Patterson. Still, the major thing I remember from solving the six site mercury derivative was the sense of desperation that came with computing in the evenings and trying to make sense of the vector map. (I think desperation is a major driving force in research. Sometimes, you just have to get things done.) Subsequently, Lyle complimented me on being able to work through that noisy Patterson map, so I suppose I must have shown a bit of skill in doing it.
In 1975, a low resolution structure of a protein was a significant result, and because the hemerythrin subunit is a four-alpha-helical bundle, there was a lot to be said about the molecule at low resolution. It was time to write my dissertation.
Once again, I benefitted from having Ling, Verner and Lyle on my thesis committee. Without any special effort on my part, the three of them disagreed on what I should include in my thesis. Should I just staple my small molecule structure papers together and call it a thesis? Should I just write up the hemerythrin structure? Should I combine all of those in a larger volume? When they disagreed, I negotiated with Lyle and ended up writing up just my hemerythrin work, about a year’s worth of my PhD research.
Writing a dissertation in 1975 was considerably different from the current process. First, you had to find a typist who was careful and skilled enough to meet the formatting standards of the Graduate School. The margins were checked with a steel ruler, and edits had to fit within the margins, so re-typing had to be minimized, especially since we paid by the page. Of course, typographical errors had to be avoided, and spell-checking was a human-based process, not a button in a word-processing program.
Since it was such an involved process, Larilyn and I decided I should go first, so three months before supporting her with her dissertation, I wrote my thesis and got it typed up. It was 71 pages long, and 25 of those were Calcomp plots of the hemerythrin electron density map. We had to submit our theses for approval before scheduling our thesis presentation, and during the two-week waiting period, I built a balsa wood model of the low resolution electron density map. (See photo below of Lyle holding the model).

Lyle Jensen
When the big day arrived, I was a little nervous. I actually wore a tie and jacket for the presentation. And after I started talking, I realized I was the expert in the room on my structure, and there was little anyone could do to keep me from getting my PhD. After all, if they didn’t approve, it would look bad for my supervisory committee. And besides that, Lyle wouldn’t have approved scheduling my final exam if he didn’t think I could handle it.
But there was still Verner. Hemerythrin is an octamer with 422 symmetry relating the subunits. I’d built a couple models of the possible subunit arrangement using Styrofoam balls for the subunits. At some point in the presentation, I held up one of the models and stated the symmetry was 422. Verner immediately asked, “What’s the symmetry of that model?” Gasp… I quickly answered that the symmetry was higher than 422 due to the spherical balls, and I said I didn’t have time right now to figure out the actual point group. And I moved on with my talk. And amazingly, Verner let me go.
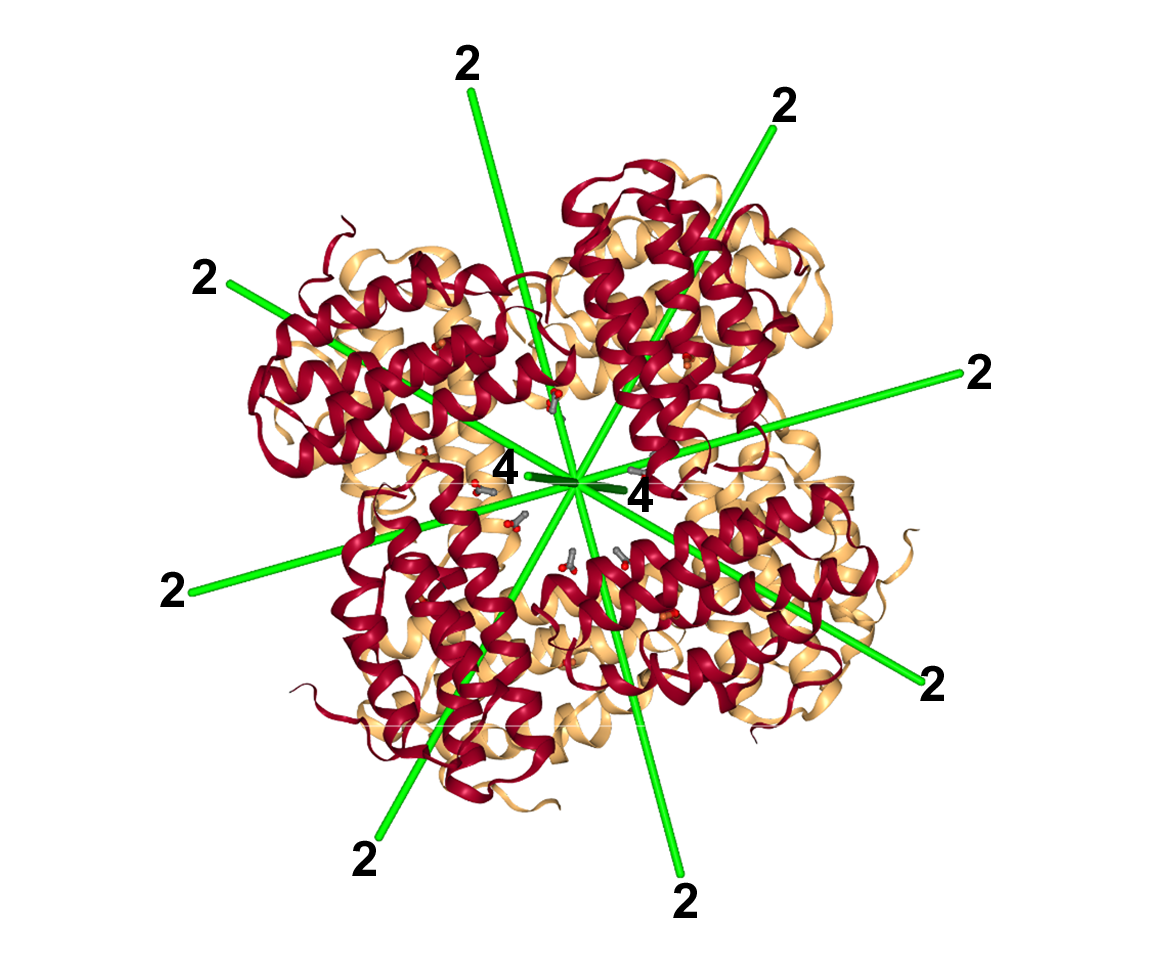
Hemerythrin octamer
I’ve thought of that moment often over the years, and I still think of it as a sort of personal triumph. It signified a time when I was the expert in the room. No one knew as much about my structure as I did. I was invincible (that day). The next day, I started on my path to being more and more confused by things, both scientific and not. I’ve heard many people, especially Lyle, Ling and Verner, talk about how they just didn’t understand this or that thing. With the exuberance and certainty of youth, I thought they must be crazy. They obviously knew more about those things than any of the rest of us did. I now understand what they meant. I will always have more questions to answer. It’s part of what makes us scholars and scientists.
Three months later, Larilyn finished her dissertation. I’ve greatly appreciated how much our shared graduate school experience and problems has strengthened our relationship. I’m also very grateful for it.
So, in the fall of 1975, we loaded a truck and drove to start my post-doctoral research back east.
|




