- History Home
- People, Leadership & Service
- A Legacy of Excellence
- History & Impact
- Meetings Through the Years
- Resources
Interviews - William L. DuaxBiography | Publications | Curriculum Vitae | Videos | Slides | Interviews | Articles | Obituary An Interview with William L. Duax By David Zierler August 20, 2020 Location: Video Conference DAVID ZIERLER: Okay, this is David Zierler, oral historian for the American Institute of Physics. It is August 20th, 2020. It’s my great pleasure to be here with Dr. William L. Duax. Bill, thank you so much for joining me this morning. WILLIAM DUAX: It’s my pleasure to be here. I appreciate this opportunity. It has caused me to look over my background, life, etc. And, it’s something I needed to do anyway. ZIERLER: Absolutely. And I’m glad to hear that. Okay, Bill, so to start. Would you please tell me your title and institutional affiliation? DUAX:I have a position at the University of Buffalo where I am one third of a full professor. Three of us at the Hauptman Woodward Institute (HWI) make up one full professor in the Structural Biochemistry Department of he Medical School. Vivian Cody, Bob Blessing, and I are one full professor. I have been at the HWI (formerly the Medical Foundation of Buffalo- MFB) for almost 50 years where I was the head of the Molecular Biophysics department (1970-1988), research director (1988- 1993), Executive VP (1993-1999) and professor emeritus, Herbert Hauptman Distinguished Scientist(1999-present). When they wanted to retire me as a distinguished scientist I asked Herb, “Could I be the Herbert Hauptman Distinguished Scientist?” And he said, “Sure.” My main activity these days is teaching a program in evolution to high school students. The student program also pleases HWI because many of the students in the program have been children, nieces, and nephews of the members of the HWI board of Directors who have benefited from being in the program. I was CEO of the American Crystallographic Association (1988-2019), president of the International Union of Crystallography, IUCr (2002-2005), and the founding editor of the Newsletter of the IUCr for 25 years. ZIERLER: Bill, when did you go emeritus from the school? DUAX: About two years ago. I no longer have a laboratory. ZIERLER: Well, Bill, let’s take it all the way back to the beginning now with your childhood and even before that. Let’s first start with your parents. Tell me a little bit about your parents and where they are from. DUAX: My mother and father were both born in Chicago. My mother had Irish ancestry. I think it’s probably her grandparents who immigrated from Ireland. And her parents were lace curtain Irish in Chicago. She began college at DePaul University, but did not finish. That is where she and my father met. My father’s parents came from Wisconsin when my grandfather decided that he didn’t want to be a farmer for the rest of his life. He would rather go into show business. And since that was just the beginning of radio, he thought that radio was going to be a good thing. So, he moved his family to Chicago and he took singing and dancing lessons. And applied for a job at WLS. He got onto the WLS Barn Dance, which was a precursor to the Grand Ole Opry. Because my great grandfather had been a honeybee keeper, my grandfather knew a lot about honeybees. He went on WLS as Bob White doing bird imitations and answered questions about honeybees. Bobwhite was the bird imitation he did best. He became known an authority on honeybees. Soon he began keeping bees in Northern Illinois. He and my father eventually had 500 colonies of bees and marketed their honey in the Piggly Wiggly grocery store chain, that became the Eisner grocery stores, that are now Kroger. Our honey, which I helped bottle and process as a 10-year-old kid was being marketed by one of the largest grocery chains in Illinois. When Illinois needed to have a chief bee inspector, my grandfather a famous authority on honeybees was given the political appointment. Donald Trump got elected president of the US because he became famous on TV. My grandfather got to be the chief bee inspector because he was famous on radio.
I was the youngest of the four generations of beekeepers shown here in about 1942.
ZIERLER: [laugh] DUAX: My brother and I continued to raise bees when I was in graduate school. We had 100 colonies of bees. Here in Buffalo, I have just two colonies of bees. I just extracted this year’s honey crop of 180 pounds of honey. My sister says, “What are you gonna do with it?” Well, I tend to give it to my friends, and they appreciate it. Bees are a recurring theme in my life. My uncle Robert was the basketball coach at St. Ambrose College and the basketball team was the Ambrose Bees. My Uncle appointed me basketball manager of the Ambrose Bees. St. Ambrose is the patron saint of bees, because his loquaciousness was attributed to his being stung on the tongue by a bee. I have never been stung on the tongue by a bee, but I usually get by with my presentations. ZIERLER: Now, Bill, you were born in Chicago, but you were not raised there. DUAX: I went to a Catholic grade school in Chicago until I was in about fourth grade. My father was a very hard worker, but he didn’t stay in any one place too long. He had a lot of different jobs and a time came when he had to leave Chicago. He put all of our belongings into a big truck and we drove 75 miles south to Ashkum, Illinois, (population 400) where I then began fifth grade. The people of Ashkum felt that anyone who came from Chicago was a gangster. It was hard to make friends. Particularly because we were living on Main Street in one section of a building with plate glass windows. It had been a shop, now turned into our home.
The building on the Main Street of Ashkum, Illinois that my Grandfather
bought in 1944 to house the Duax Honey Company. For a couple of years we lived in one of the storefronts.
We were considered to be white trash gangsters from Chicago. And I’m sure that that had an impact on my overall attitude toward life. My mother was determined that her children should go to college. She did not complete college, nor did my father. But, she put me into a Catholic high school 20 miles north of Ashkum that had good college prep courses. I always enjoyed acting and performing. I was in plays in grade school, high school, and college. When I started college I thought I would like to be either a writer or an actor. And as it happened, I’ve managed to do some of those things in my private life. In college I took courses that would make it possible to pursue a carrier in science or business. One day the professor of chemistry, James Resnick, asked me if I had declared a major. And I said, “No.” And he said, “Well, why don’t you declare chemistry?” And I said, “OK.” So, that’s how I became a chem major. I had not been involved in student government until my junior year when I ran for president of the student body. The year before there had only been one candidate. I thought that in a democracy there ought to be at least two candidates, so I was gonna be the second candidate. At the time, I worked in the kitchen clearing dishes and garbage at a window where all the students brought their tray full of dirty dishes. I put a sign above the window that said, “Get Duax out of the garbage and into the White House.” That got me elected student body president of St. Ambrose College. ZIERLER: Now Bill, I want to ask. When you were growing up, did you demonstrate any particular aptitude for the natural sciences? DUAX: No, I had demonstrated an aptitude for singing and dancing and performing. I did an imitation of the Woody Woodpecker song when I was maybe four or five years old. And everybody applauded and laughed. And I thought, “Well, I must be good at this.” I didn’t realize that I was just a funny little fool. But I got encouragement for that. I believe that a lot of actors are successful because they grow up with inhibitions, are shy and don’t interact well with others. But when they act in a play, they’re somebody else. Suddenly they’re freed of all their inhibitions and are able to just express the feelings of the character they’re playing. When working with actors in local performances, I see shy people overcome their shyness by being somebody else. ZIERLER: So, when you were thinking about college, you were not thinking at that point about pursuing a career in science. As you said, that only came later on. DUAX: My uncle was the basketball coach and athletic director at St. Ambrose College and offered me a position of team manager. I washed the jockstraps every night for my tuition, got a job in the kitchen to cover my board, a job in the chem department doing sewer analysis to pay for my room and then a part-time job cleaning an office for spare change. After my freshman year I only returned home to visit. ZIERLER: Bill, when did you realize that you were good at science? That you did have this aptitude and that you could make a career in this field? DUAX: In graduate school. When I graduated from St. Ambrose, I did not want to wait and be drafted. It was after Korea, but before Vietnam. Friends decided the best thing to do would be to join the Army Reserves. I joined the reserves, did six months of active duty, six years in the Reserves and got my honorable discharge. When I came back from the six months training, a friend of mine, Ralph Newhaus, said, “Why don’t you go to graduate school at the University of Iowa? You can probably get an assistantship.” If I was gonna get paid to go to school, what could be better? I got a teaching assistantship in the chemistry department at The U of I. I enjoyed the teaching, but I needed a PhD project and a major professor to guide me. When I talked to a nice, young professor doing quantum chemistry, I didn’t understand a word he said. And so, I said, “Well, that’s out.” Then I spoke to Norman Baenziger who was an x-ray crystallographer. And he told me that if I studied a crystal, I would learn the arrangement of the molecules in that crystal. I would be the first person who ever really “ saw” that molecule. And no one would know more about it than I did. It would be easy to defend my thesis and get my PhD. So, I was drawn into the beauty and reliability of X-ray crystallography. You recently interviewed Helen Berman and at the very end of her interview you discuss why crystallography is special. Why you collect so much data, and if the data all fits together then you really can trust the result that you get. ZIERLER: Right. DUAX: It is the most reliable type of data. And that’s why the Protein Data Bank (PDB) and the Cambridge Structural Database (CSD) of small molecules and all other crystallographic data bases are considered to be treasures for accuracy. There are no alternate facts. There aren’t two different crystal structures that have the same cell dimensions. We deal in real irrefutable facts. So, I was attracted to crystallography. Baenziger applied to NASA to get a fellowship so I could work on a specific problem and didn’t have to teach anymore. I loved teaching but I couldn’t turn down this opportunity. So, I began to work on my first crystal structures. One of which has never been published. I didn’t quite finish it. I have published plenty of manuscripts. Right now, there are some papers that I need to write concerning the evolution of the genetic code. Those are the next publications I really have to complete. ZIERLER: Bill, in what ways was your dissertation topic a result of what your dissertation adviser was working on? And how much of it was your own interest and what you wanted to study yourself? DUAX: It was something my advisor thought would be a good thing to do. But, it wasn’t something that he had ever worked on. The Japanese had published some papers about the thermal motion of molecules of carbon disulfide in the solid state. Molecules of carbon disulfide vibrate, rotate, and translate in the same way in solid, liquid and gas state. The Japanese used Raman and infrared spectra to determine the vibration-rotation parameters of carbon disulfide in frozen solid crystalline powder. Baenziger said, “It would be nice if we would look at the thermal and molecular movement of molecules in single crystals of carbon disulfide and see if they agree with the measurements by Raman and infrared spectroscopies.” We were trying to bring together data from crystallography and data from Raman and infrared spectra and see how well they match. He challenged me grow single crystals of carbon disulfide and analyze its thermal motion.” It was the first time that such a project was ever undertaken. I grew the crystals and determined the structure. We found that the crystallographic, Raman and infrared data were in full agreement. The results got me a lot of attention because it tied together consistent results from different disciplines in science. Once again, it wasn’t one set of facts for Raman and infrared and a different set of facts for crystallography. They matched. That was very good for both disciplines. ZIERLER: Bill, when you talk about the value of this research and the attention that it garnered, are you referring to its value just in the sense of basic science or were there practical applications of these discoveries at play as well? DUAX: At a time when some people question the value of science I think it is important to know that there is agreement within science and that there are facts that are irrefutable. I think that the more valuable contribution in this case is to basic rather than practical science. My studies of estrogens and anti-estrogens is a practical application. Estrogens support cancer. Anti-estrogens battle it. Finding subtle differences between the structures of estrogens and anti-estrogens can contribute to the design of more potent drugs to treat breast cancer, a very practical application. When my students study the proteins of mycobacterium tuberculosis, we look for proteins that are not present in other genomes that can be suitable targets for drug design. We look at the evolution of protein molecular structures in order to identify, and target the right member of that large family that may extend all the way from the earliest bacteria to us? We do not want to cause “side effects” that might occur if a poorly designed drug interacts with other members of the target proteins family. ZIERLER: Bill, as a graduate student, were you thinking about the applications to human health research? Was that something that was specifically compelling or motivating for you? DUAX: As soon as I had my degree in x-ray crystallography, I wanted to use this powerful technique in biomedical research. ZIERLER: And so, what did you end up doing after you defended? DUAX: I got my first postdoctoral position with Abraham Clearfield in Athens, Ohio. He needed a crystallographer to determine the structures of molecules that formed infinite sheets that could trap radioactive ions in waste products from nuclear reactors. I had to grow crystals in an oven inside of a sealed glass bomb. I had to break the bomb open, find crystals and determine the structures of these layered compounds. It’s been 50 years since I did this research, or I would be able to explain it better. While I was working with Clearfield, Baenziger suggested I apply for a position at the Roswell Park Crystallographic Institute where the first protein structure had been solved. I applied and was invited to come for an interview. It turned out that the woman who interviewed me, Dorita Norton, was in the process of moving from Roswell Crystallographic Center to the MFB. She had a grant to study the structures of steroids involved in breast cancer, prostate cancer, heart disease and other steroid connected diseases. Dorita won me over immediately. I wanted to go to this MFB and begin to work on structures of steroids. I believe that our work on structures of estrogens, androgens, and progestins did make a contribution.
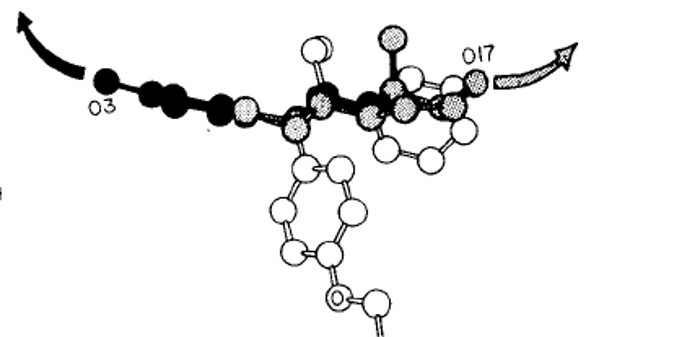
Crystallographically determined structures of the natural estrogen estradiol (shaded atoms) and the anti-estrogen tamoxifen (unfilled atoms). Tamoxifen is the most commonly used drug to combat breast cancer. Estadiol and tamoxifen have a phenolic ring in common (black atoms). The phenolic rings of both molecules bind to the receptor. The hydroxyl (O17) of estradiol sustains a conformational change in the receptor but the bulky tamoxifen prevents the receptor from stimulating cancer growth.
All steroid hormones act through a receptor mechanism. When an agonist binds to a steroid hormone receptor the receptor functions. If an antagonist binds to the receptor it blocks the function. We developed a model for how all the steroids function. Our structural data suggested that the A-ring end of the steroid initiated receptor binding and the D-ring end distinguished agonistic from antagonistic action. By interacting with the receptor in the lock and key relationship the steroid can either turn a process on or off. 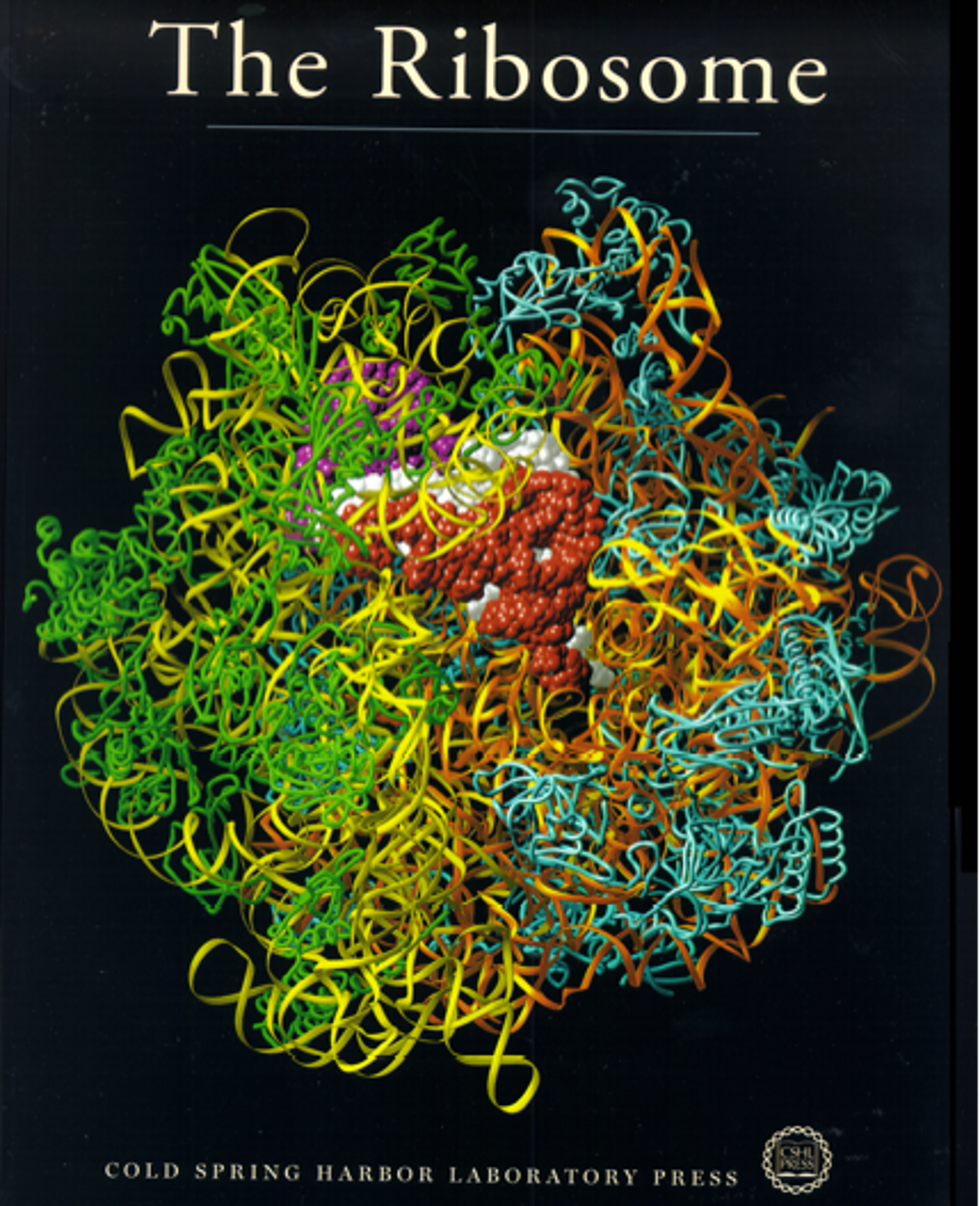 Green and blue ribbons are the ribosomal proteins of the small and large subunit. Yellow and orange ribbons are ribosomal RNA. The space-filling molecules are the transfer RNA molecules shown in the three positions they occupy during the process of adding specific amino acids to the protein being synthesized. They are the entry (red), peptide formation (white) and exit (purple) sites.
ZIERLER: Bill, did you ever think at this point about pursuing a medical degree? Or becoming more closely involved with clinical research? DUAX: By the time I was at the MFB I was married and had four children. And I wasn’t about to go back to school. My father said I had spent too much time in school all along. ZIERLER:[laugh] DUAX: I’ve known a couple of remarkable people with MD-PhD degrees, who had wonderful careers caring for patients and advancing medical knowledge. I’ve had student programs at MFB/HWI for almost 45 years. I would bring in a class of high school students and show them what crystallography was about and then they would have lunch with Nobel Laureate Herbert Hauptman. If the students were interested in medical science I would urge them to consider a PhD-MD program ZIERLER: Bill, I wonder if you could talk a little bit about your early impressions of HWI? What was it like? In what ways was it unique from, say, a teaching hospital or a university affiliation? DUAX: At the time, I wouldn’t have been able to contrast HWI with other institutions. I was impressed with the fact that we were using crystallography to determine hormone and antibiotic structures. I knew how important the structures were. I knew that there was a department of crystallography in Pittsburgh. But that department was not solely devoted to biological research. It was more involved with the industrial community of Pittsburgh. And the director of that program, George Jeffrey, did very well in developing close relationships with the entire industrial community around Pittsburgh. Helen Berman, and several of the other people that she worked with through the years all got some of their training at the University of Pittsburgh. Jeffrey, himself, was an authority on sugar structures. I also got to know Jenny Glusker who worked on the structures of carcinogens in Philadelphia. I was pleased that at MFB/HWI, we studied steroids, which had a wide range of applications. There were two other departments at HWI. One, in thyroid chemistry and the other in basic endocrinology. So, I was learning about the field of endocrinology. When I went to my first Endocrine Society meeting, I was stunned by the decisions that would be made on the basis of a small number of pieces of data. Somebody had three rats; one ran away and the publication concerned the other two. I felt that research in endocrinology lacked in the type of reliability present in crystallographic studies. Vivian Cody was reporting about her studies of thyroid hormones at endocrine society meetings. We were the only two people at Endocrine Society meetings who were talking about the molecular structure. It was fifteen years later that the Journal of Molecular Endocrinology came into existence. Our early reports of the role of the role of molecular structure in hormones/receptor interaction enhanced our reputation at the Department of Arthritic and Metabolic Diseases of the NIH. In the early days we were determining our structures with the heavy atom method. If a hormone or antibiotic with a ”heavy atom”(usually bromine) attached to could be crystallized it was possible to determine the location of the “heavy atom” in the unit cell. The entire structure could be found by studying the electron density around the heavy atom to boot strap a complete determination. The heavy atom technique was developed by David Harker at the Roswell Park Crystallographic Center in Buffalo and by Max Perutz, in England, while each was working on one of the first proteins to be studied by X-ray crystallography. The heavy atom technique was used to determine all of the earliest protein structures. Herbert Hauptman came to the conclusion that you can determine structures without heavy atoms by looking at the relationships among the reflections that are most strongly diffracted in the x-ray pattern. The strongest spots (reflections) in an x-ray pattern are those spots coming from planes that have the maximum density of electrons in them. The relationships between the most intensely diffracting planes can be used to assign relative phases that locate probable atomic positions in the unit cell of the crystal. Hauptman and Karle developed mathematical relationships and techniques based on probability methods to predict phase relationships. Hauptman and Jerome Karle tried for 20 years to explain direct methods to crystallographers at ACA meetings where they would be ridiculed. David Harker insisted that they were wrong. When David Harker was once confronted with determining the structure of a boron-containing molecule for which a heavy atom derivative could not be synthesized. It was a borohydride and boron’s chemistry was unknown at that time. Harker and his collaborator, Kasper, noticed that some of the reflections had interesting relationships to one another. They termed these to be inequality relationships and they used their interrelationships to determine the crystal structure. Hauptman realized that Harker had proved that the diffracted intensities contained information about their relative phases. Prior to that, it was believed to be impossible to determine phases from intensities only. You could gather the intensities of the data, but you could not determine their relative phases. ZIERLER: And what’s the significance of that, Bill? DUAX: You could solve structures without heavy atoms. When we were using heavy atom derivatives to determine steroid structures, we co-crystalized the steroids with bromophenol. We got bromine into the crystal without putting it on the steroid and we learned that we could crystalize complexes. Herbert Hauptman developed a way to determine crystal structures without heavy atoms. Dorita Norton began collaboration with Hauptman. Hauptman while he was at the Naval Research Lab where he and Jerome Karle were developing direct methods. Hauptman got his PhD and for a while, there were two programs at the Naval Research Lab working on direct methods. Jerome Karle’s program that had been there for years, and Herb’s program. But the Naval Research Lab decided that they didn’t really need two direct methods programs. They told Herb they wanted him to turn the laser into a killing weapon. And Herb did not have any desire to go from direct methods in structure determination to turning the laser into a killing weapon. The offer from Dorita Norton to come to Buffalo to continue direct methods relieved him of the need to turn the laser into a killing weapon. ZIERLER:[laugh] DUAX: And so, Herb came to the MFB where I was the head of the crystallography department and he became a member of my department. It’s crazy that I was the head of a department with Herb Hauptman as a member. ZIERLER:[laugh] DUAX: But we worked well with the help of computer experts Chuck Weeks and Steve Potter. Herb and I were both a loss at computers. Herb was a mathematician and a master of probability methods. My talent, if I had one, was in pattern recognition. I could see patterns of interactions between the strongest intensities in the data set that indicated which planes contained the highest concentration of electron density and atoms in those planes. ZIERLER: And Bill, can you describe what’s the value of pattern recognition, generally? What does it allow you to do? DUAX: It allows you to look at the hundreds of thousands of proteins in the gene bank and put them into families and classes by looking for patterns that are conserved in all members of a family. The ribosome is present in all cellular species. The genomes of all cellular species contain the sequences of from 50 to 70 ribosomal proteins. We are able to find at least 30,000 members of each family of ribosomal protein sequences. And each one of the ribosomal proteins will have a signature pattern that is retained in all of them. Although the entire sequences will have very little homology, if you line up all members of a specific ribosomal protein family, it will have a few fully conserved key residues. Most of the time, the key residues include glycines and prolines. We have discovered this by analyzing families of ribosomal proteins. Finding a specific pattern of around 10 amino acids in 10 specific positions in the alignment of a family of proteins, allows us to place them all in the same family. Pattern recognition, and bioinformatics can catalogue protein families and super families. If a protein is a drug target, we can examine similarities and differences that can be used to design specific drugs. ZIERLER: Bill, can you talk a little bit about the funding sources for HWI? How does it operate? DUAX:HWI has a small endowment, to small to retain all the staff. When Herb came to MFB, Dorita Norton had NIH funding for the steroid and thyroid research projects About this time prostaglandins became popular, and so we put together a proposal to study them that got funded. The NIH had a policy for funding shared resources for data collection. Because we had three programs funded to study crystallography of hormones, we were able to apply for a shared instrument. Usually a shared instrument would serve several institutions. But, since we had so many projects in crystallographic structure determination at the MFB, we were able to get a shared instrument. At MFB I put Doug Rohrer in charge of the shared instruments to see to it that all the users would have equitable access to the shared instrument. Doug ran our shared instrument very effectively. Soon it was time to begin to determine the structures of proteins targets of the hormones including enzymes involved in their synthesis. The steroid project was funded for 30 years.
Dorita Norton
Shortly after Herb came to Buffalo, Dorita Norton tragically committed suicide. Herb called me one Sunday morning to tell me of her suicide. I had been with her the day before in the hospital. She underwent an operation for breast cancer. And she was told that she was okay, but she didn’t believe it. When I went to visit her that day, we talked for about an hour. She went through all the staff and told me how I really needed to be more supportive of this person, and I really should watch out for that person. She was telling how to take care of the staff after she killed herself. The next morning when Herb called me and told me she had shot herself in the heart, we tried to figure out how we were going to salvage the institute. ZIERLER: Meaning that her death was so… she was central to the institute that it posed existential questions? DUAX: She was the principal investigator on the two grants that were the primary source of support for the institute. ZIERLER: Yeah. DUAX: Herb took over the thyroid grant and eventually transferred it to Vivian Cody. I became PI of the steroid grant. She had been the principal investigator. The people in Washington were very understanding, very sympathetic. We had very good relations with the NIH head of the area that was responsible for our grants. And we maintained and built upon those relationships through time. ZIERLER: Bill, in what ways were you and Herb able to fill her shoes on these ongoing projects? And in what ways were you not able to? DUAX:I had been running the steroid project since Tony Cooper’s departure. ZIERLER: Yeah. DUAX: There was no difficulty in taking it over. I was writing the papers and giving most of the talks. If Dorita had to give a talk, I would feed her all the information she needed. Similarly Vivian Cody was really doing all of the work for the Thyroid project. Dorita was the PI, but she was not engaged in the day-to-day activities. Originally Dorita had hired an Australian crystallographer named Tony Cooper to determine the steroid structures. He was trained in Dorothy Hodgkin’s lab when Dorothy received her Nobel Prize. He got the telephone call from Stockholm because Dorothy was in Ghana. At MFB Tony was solving the structures with the help of a graduate student, Chuck Weeks. But he found working for Dorita intolerable. The salary was okay, but he was frustrated and unhappy. When he left, Dorita put me in charge of the project. From then on, I was solving all the structures with the help of Chuck Weeks and Steve Potter. When Herb came we got as interested in advancing direct methods of structure determination as the structures themselves. By 2016, we had gotten grants from the NIH totaling $48 million spread over a dozen different PIs, Thirteen million of that went to Hauptman for his direct methods development. Ten million was in the steroid project with me or the ion transport antibiotic project that I also developed. We were very successful in getting competitive grants from the NIH over a long period of time. That’s how we stayed alive. The valinomycin structure determination was another important accomplishment. We were using direct methods to solve the steroid structures but we wanted to do larger structures. A sample of an antibiotic called valinomycin was given to us by Charles Winter, a scientist at the Roswell Park Cancer Institute. When we determined the structure of valinomycin it was the largest structure solved by direct methods at the time and it had crystalized in the space group P21. X-ray diffraction data reveals the dimensions and symmetry properties of the unit cell. The intensities of diffracted data reveal the location of atoms in the unit cell if the relative phases of the diffracted data can be determined. Crystal structure determinations are easier to phase in centrosymmetric space groups because all phases in them are either 0 or 180 degrees. In non-centrosymmetric space groups only a few of the reflections have phases restricted to 0 or 180 degrees. Most phases in non-centrosymmetric space groups can have any values from 0 to 360 degrees. The monoclinic space group P21 and the orthorhombic space group are non-centrosymmetric space groups in which most drugs and hormones crystallize. In space group P212121 reflections in the HK0, 0KL, and HOL planes have phases of 0 or 180 allowing a choice of origin that fixes the enantiomorph and can be expanded to phase other reflections. In space group P21 reflections in the H0L plane can phased to fix the origin but not the enantiomorph. When we determined the structure of valinomycin it was the largest structure solved by direct methods at the time and it had crystalized in the space group P21. While trying to phase the data for valinomycin I discovered that half the data in space group P21 is enantiomer specific and half is not and figured out how to separate the two halves and achieve strong enantiomorph selection. Using strong enantiomorph selection I was able to determine the structures of the principal mineralocorticoid steroid aldosterone and the antibiotic valinomycin. Herb and I wrote two manuscripts on the valinomycin structure determination. They are two of my favorite papers because Herb and I are coauthors on them.
Determining the structure of Valinomycin in 1972.
ZIERLER: Bill, beyond the coauthorship, what is it substantively about these papers that is also so important for you?
DUAX: I was pleased to have I found a way to define the enantiomorph in the space group P21. It was a relationship in P21 data sets that had not been previously detected and it could be used in other structures determinations. The most important research proposed in the ionophore grant was the determination of the structure of gramicidin A, a large complex sodium specific ion transport antibiotic. David Langs was a fine MFB scientist. He died recently. David was brilliant practitioner of direct methods. I gave the gramicidin data to David Langs and I said, “David, only you can solve this structure.” David did solve the structure. Some of the solution may have been mystical. He was pretty mystical when he talked about it. But he did solve the structure. When the structure was published my name did not appear on the publication. My grant was acknowledged, but my name was not there. My NIH project manager wanted to know how could that be? Why wasn’t my name on the paper? I told him, “Because David Langs did all the work and he deserves all the credit. I think David appreciated it. I certainly appreciated him. ZIERLER: Bill, can you talk a little bit about the quality of the instrumentation at HWI? Did you get a sense that you were working with state of the art equipment? DUAX: Yes. The shared instruments that we applied for were state of the art equipment. When I came in to the institute I worked on a diffractometer that Dorita Norton had brought from Roswell Park. You had to set three dials, push a button, and record an intensity. Then you had to set three more dials and push the button again. It seems tedious now, but at the time it was wonderful. I didn’t go to lunch. I worked all day long gathering data because the data was so easy to get. When we applied for shared instruments, they were state of the art. When we solved the valinomycin structure, an additional complication arose. Scientists at the Shemyakin Institute in Moscow were international leaders in the field of peptide synthesis. They published a paper in Science about complexed valinomycin in which they said that there was the only way that valinomycin could fold. When we determined the structure of un-complexed valinomycin we found a different conformational fold. By comparing the structures of complexed and un-complexed valinomycin we proposed a mechanism for ion complexation and membrane transport. Science was very pleased to publish our paper in which we stated that the Russians were wrong when the said that there was only one possible conformation of valinomycin. I thought Science published the paper because of its scientific content. I did not think it was valued because it criticized Russian work during the cold war. It’s only in the last couple of years that I’ve thought this through. Yuri Ovchinnikov, who was a vice president of the Russian Academy of Sciences, came each year to meetings of the Peptide Society.in New York City. When I saw that he was coming to a Peptide Society meeting I invited him to Buffalo and give a talk. And he said he would be happy to come. I didn’t know he was the vice president of the Russian Academy of Sciences. I just knew that he was somebody who worked on the spectroscopy of valinomycin. When he arrived I picked him up at the airport and brought him to a reception at my house. I offered him wine and asked, “Would you like white or red?” His response was “Red, of course.” He was a tall, handsome, and gregarious man. He enjoyed coming to the U.S. He would go back to Russia bringing magazines, comic books, and souvenirs to his staff. The next day I questioned Ovchinnikov about what I should say when introducing him. When he told me that he was the vice president of the Russian Academy of Science, I thought “Oh boy, this guy’s really important.” By the time we go downstairs to our very small seminar room, the hall was packed with people who knew who he was even though I didn’t. After his talk Herb and Edie Hauptman and my wife Caroline and I took him to lunch. At lunch he invited all four of us to come to Russia at his expense. He said he wanted to start collaboration with us on methods of structure determination. He had wanted to start a collaboration with Jerry Karle. But that was impossible because Karle worked at the Naval Research Lab, which was off limits for Russians. So, he wanted to collaborate with us. He went back to Russia and purchased five $50,000 Syntex diffractometers and put them in five institutes in the USSR. When I visited later he had me go to each of those places and give talks on how to use the data they were collecting. Ovchinikov sent his colleague Vladimir Pletnev to Syntax in California to learn to collect intensity data. Then Ovchinikov sent Pletnev to Buffalo to learn Hauptman’s direct methods. Ovchinikov brought modern X-ray crystallography to Russia. And it all started with a paper about valinomycin. In front of the Shemyakin Institute in Moscow, there is a huge iron sculpture of valinomycin.
David Langs, Vladimir Pletnev, Bill Duax and Caroline Duax in front of iron sculpture of Valinomycin in Moscow.
ZIERLER: [laugh] DUAX: And along one side of the Shemyakin Institute is the place where Linus Pauling and Dorothy Hodgkin planted trees. The trees haven’t survived, but the monument has. And Pletnev, although there were some ups and downs, is back at the Shemyakin now where he continues to run the crystallographic group. Oh, we’ve had controversies with them about the conformation of valinomycin, yada, yada, yada. No point in going into that. But this was how I got engaged in more international activities. And in particular strong…Vladimir Pletnev is a good communist, but I think he basically has a belief in religion that he hid all the while until communism collapsed and he could go back to church again. Which disappointed me because I didn’t see why he would want to go to church! Anyway, Pletnev is one of the people in the scientific community that I have the greatest confidence in. And trust. I would like to trust all the collaborators I’ve ever had, but that’s not realistic. ZIERLER: Bill, I want to go back a little in the chronology and talk about your work at SUNY Buffalo. So just to be clear, when you joined HWI in the late 1960s, SUNY Buffalo was not part of the consideration? This was separate? DUAX: Yeah. I came to the Medical Foundation of Buffalo. ZIERLER: Right. And so, when did you join, or when did it occur to you that this would be a good thing for you to pursue? An appointment at SUNY Buffalo? DUAX: I contacted people in the Medicinal Chemistry Department at SUNY because they were developing medicines. I determined crystal structures of drugs that I received from four or five members of the Med Chemistry Department. The Med Chem Department held annual symposium. And they would bring in eight or nine scientists to talk for three or four days. I offered to run the 25th annual Med Chem Dept. Symposium June of 1984. I titled the program “Recent Advances in Techniques for Drug Design and Confirmational Analysis”. HWI had nothing to do with this. I invited 39 world leaders in the field, appointed session chairs, and allocated only 30 minutes to each speaker. The speaker’s list remains impressive 35 years later. I used a rubber duckie as a timer and the duckie never had to quack. If the duck came out on the podium they would pretty much stop in five minutes or the duck would start to quack. I still have the duck. That reminds me, I noticed that in Helen’s interview there were no figures running down the column. I will want to put figures throughout this, after we get the final copy. I will put the appropriate figures. ZIERLER: Certainly. DUAX: Before the meeting I asked Olga Kennard to send someone to show the people attending the meeting how to use the database in order to promote wider distribution. At the annual Med Chem meetings, I met a lot of people from drug companies and I had already distributed the database to some of them but there was a huge audience that wasn’t subscribing. I solicited support from 14 corporations, had 10 stations in the exhibited hall, an evening of computer films, 38 posters and a banquet at the Albright-Knox. So, it was a very splashy meeting. The subscriptions to the database rose very nicely. When the HWI became the home of the structural biology division of the medical school, they offered us two salary lines and promised four more. The four more never showed up. But the two lines that they gave to us for the structural biology department were divided into thirds. That’s how I became a third of a full professor. Bob Blessing a third and Vivian Cody a third. The other thirds went to Herbert Hauptman, George DeTitta, and Walter Pangborn. Herb died and George and Walt retired from their positions. The other two have retired from their positions. But Bob, Vivian, and I retain our one third of a position which is a nice piece of change. A university professor at UB is now making about $150,000 a year. I have no trouble making ends meet on $50,000 a year. Over the years I had graduate students, Masters degree students and post-docs from abroad. Bobby Huether was in graduate school in computer engineering at SUNY when he came to work with me part time analyzing the evolution of proteins. He found bioinformatics more exciting than computer engineering. Soon he began doing protein crystallography with Tim Umland one of my colleagues at HWI and got his degree. When he went out to get a position and could show that he was experienced in protein crystallography and bioinformatics, he got a $4,000 signing bonus. And he’s gone on to have a successful career, moving more and more into bioinformatics. He’s in a laboratory in Chicago right now. Being skilled in more than one discipline is a very important factor in today’s scientific community. ZIERLER: Bill, it sounds clear. Even if it wasn’t part of the original plan coming to Buffalo, that this appointment at SUNY Buffalo was absolutely critical for your own research. DUAX: Yes it has been. I’m fortunate to have a 50-year connection with crystallography, it’s always been the center of my research and activities in the ACA, the IUCr, and the CSD. All of these things grew out of the HWI focus on crystallography and endocrinology. With support from the Library of Medicine we published two volumes of The Atlas of Steroid Structure in which we presented comparative analysis of all the steroids and an introductory section to help endocrinologists understand and use the information. At that time Olga Kennard was gathering the data on all small molecules for which crystal structures were reported into the Cambridge Structural Database (CSD). We learned from each other. To support the CSD, Olga sold copies to dozens of different countries. England did not have to pay anything because they had provided the support to her lab to get the Cambridge Database organized. The NIH paid for the US copy. The NIH had some staff who were to provide a copy of the database to any academic in the U.S. who wanted it. But they weren’t getting the job done very effectively. Many drug companies had in house crystallographers and they did not release or share their crystallographic data. Olga was charging the for profit community $5000 per copy because they withheld their data and they were for profit. The drug companies in the US were using Fortran and Cambridge was not able to produce copies of the CSD in Fortran. Olga and I met at a meeting, and she asked, “Do you think you could distribute the database in Fortran?” I said, “Yes.” I wasn’t sure we could, but I felt confident that Chuck Weeks and Steve Potter would figure out how to translate the database files into Fortran. And then our colleague Phyllis Strong would make copies and send them out. So, we immediately began distributing copies to the for profit community, charging them $5,000. And Olga let us keep $1,000. But each year, the price went up. She got more money from the drug company for a copy and she paid us less for making the copy. That’s the way finance works. And Olga was brilliant at it. Eventually, the academic community in the U.S. learned that we were distributing copies of the database in an effective manner. And they were willing to pay us $500 per copy instead of waiting to see whether the NIH would ever get around to sending their copy. So, we began being the distributor to the academic community and was another source of income for us. That went on for a while until Cambridge decided they could do everything from England and cut out the subsidies that they were getting from countries or subsidies from us for the for-profit community. So that was the end of that source of revenue. But each time there was a loss, we would scramble around and figure out where do we go from here? But it’s always been connected with crystallography. ZIERLER: Bill, I wonder if you could talk more broadly about some of the advances that have been made in steroid structure over the years? What are some things that were big question marks when you got involved? What’s understood really well now? And what might be some ongoing mysteries with regard to steroid structure? DUAX: We had this model for steroid function proposing that the A-ring bound to the receptor and the receptor changed shape, stabilized by the D-ring. That was our A-ring binding D-ring model. But you couldn’t really test that model unless you had crystal structures of the receptor with agonists and antagonists bound to it. So, it was essential that we start to do protein crystallography at HWI. I went to guy Dodson’s lab England and spent some time getting some basics, but I never really learned to be a bona fide protein crystallographer. When I came back to Buffalo after a sabbatical I hired a trained protein crystallographer, Debashis Ghosh [Fig 9], to work on protein structures at HWI. At that time the receptors were not isolated and purified well enough to be able to undertake a receptor structure. There were enzymes that modified or metabolized steroids including dehydrogenase enzymes that had been purified. So, Deb Ghosh purified and crystallized and gathered the data for a couple of steroid dehydrogenases and for a cholesterol modifying enzyme. We determined the structures of three enzymes. I played little role in any of these determinations except for the first dehydrogenase where I did make a minor contribution. There were four molecules in the asymmetric unit and there was only a certain way that those four could be arranged in the crystal. Dorothy Hodgkin made some early observations based upon the cell dimensions of cholesterol that refuted claims about its structure. She said that the cholesterol molecule they proposed could not be arranged in the crystals that cholesterol formed. Cell dimensions and space group symmetry relationships can help determine how multiple copies of a protein can come together in a crystal structure. We found that the steroid dehydrogenases were members of a family that could be traced back 3 billion years to the earliest bacteria on earth. One of the very first protein crystal structures was determined by Michael Rossmann and its three-dimensional fold (a beta sheet with alpha helices on either side of the sheet) became known as the Rossmann fold. The Rossmann fold is the most common fold in the gene bank. Subsets of the Rossmann fold superfamily have distinguishing glycine rich sequences near the N-terminus. We noted that the steroid dehydrogenases have a Rossmann fold with an 8 amino acid sequence [Threonine-Glycine-XXX-Glycine-X-Glycine] near the beginning of the the N-terminus. This subset of Rossmann folds is present in thousands of proteins in the gene bank. including the earliest G+ bacteria causing tuberculosis and leprosy. It appears to be the most primitive of Rossmann folds and includes a beta-keto acyl carrier protein (BetaKACP) that is critical to the synthesis of an essential membrane protein in all cellular species.
Superposition of five of the thousands of proteins having the most commonly observed protein fold, the Rossmann fold. Pattern recognition allowed us to define all members of a family. Biochemists wanted us to do additional dehydrogenases they were studying. If the enzyme had the TGXXXGXG sequence, we knew it was a member of the BetaKACP family and its 3-dimensional structure could be predicted using computational methods. We needed be determining the structures of proteins in families for which no family member had been reported. That gave us insight into two things. One, our ability to align an entire family and two, how once you align them you can determine exactly how many families of different proteins are in the entire gene bank. Right now, the estimates range from low estimates of maybe 25,000 different families to high estimates of 50,000. But that’s better than the millions of structures that are occurring in the gene bank. So, by using bioinformatics, family tracing, and pattern recognition, we should be able to identify around 30,000 families that covered it protein folding space. I was trying to persuade people by showing them that the BetaKAPC superfamily was in every cellular species on earth. I soon realized that my argument would be more persuasive if I traced the evolution of ribosomal proteins that have to be present in all genomes. Defining evolutionary tree goes back to the time of Darwin. The evolutionary trees of Darwin and Ernst Haeckel separate branches for bacterial, plant and animal species. My students and I are trying to produce an evolutionary tree based upon the evolution of ribosomal proteins. Once DNA became recognized as having the information about the sequence of all proteins Carl Woese examined ribosomal RNA (rRNA) to identify any differences between the rRNA of bacteria and eukaryota. Woese was able to separate eukaryotes and bacteria, but he had a small subset of data for which the rRNA that shared homology with both bacteria and eukaryotes. He concluded that these species were at the root of the evolutionary tree and called them Archaea. His idea that the archaea were at the base of the evolutionary tree is no longer accepted. The archaea are now believed to be a separate family that arose in parallel with eukaryotes.
The accumulation of thousands of genomes has made it possible to attempt to create evolutionary trees on the basis if the evolution of members of a protein families. Scientist aligned a protein in all drosophila or all mammals to trace pockets of evolution. Ours is the first group to looked at protein families that are present in every cellular species. Most proteins of the ribosome have the same 3D fold and approximate length in all bacterial, eukaryote, and archaea. We have been aligning all members of each family of proteins of the small subunit of the ribosome; over 30,000 members of each of 21 protein families. There are positions in the alignment in which glycine(G) or alanine(A) or arginine(R) or Proline(P) residues are fully conserved in 30,000 proteins. In print out of these alignments the fully conserved GARP residues are vertical lines that are shaded black. We like it when I say, “Black is beautiful. And if there is a sequence in the that is in error, it will show up as a white line running horizontally across those vertical black lines. We call those misfits “white trash.” ZIERLER: Black is beautiful and white trash, huh? [laugh] DUAX: Yeah. And that has been appealing to my students of color. But only one of them has ever used that terminology in her presentation. A. Marschal is a wonderful, young woman who received the Martin Luther King award from her school upon graduation, and has received other awards since. ZIERLER: Bill, maybe this is an obvious question. But can you explain the value of taking an explicitly evolutionary approach to these inquiries? DUAX: I am very pleased to be teaching evolution to these students. I had a question from an audience once when we were making a presentation. A young girl asked, “But isn’t evolution just a theory?” I replied, “Well, we don’t address the question of whether it’s a theory. We just show how it works. We show the details of the evolutionary process.” When you can line up 30,000 structures from the earliest bacteria that extend over a three billion year time, then I think you are providing evidence of the evolution of everything. I don’t know that that answers your question. ZIERLER: Absolutely. It’s beautiful. No. It’s a beautiful, simple, and elegant response. Bill, I have another broad question. Can you reflect generally on some of the technological advancements that have been made in crystallography over the years? And how that’s improved both the research and the discovery? DUAX: The process has been accelerating. The computers run faster. The data collection is faster. My first data I collected on film and had to look at the film and say, “This spot is stronger than that spot.” You had to make a way to analyze all of those spots. And then the automatic, the Geiger counting techniques. When I was collecting a data set, I came to Buffalo. I was so delighted with this much more powerful technique. When I used the counting device in Baenziger’s laboratory I collected every spot 20 or 30 times. When I averaged the 20 or 30 measurements, I got accurate standard deviations for each intensity. That was all very important if I was gonna determine the molecular motion in these crystals accurately. First I had to learn how to grow the crystals. I filled a capillary with carbon disulfide and mounted it on the diffractometer. Baenziger had figured out how slowly roll a liquid nitrogen source up to the capillary. When I did this the capillary would become filled with crystallized powder. Then I would dial the cooling machine slowly back until there were only a few crystals at the tip of the capillary. Then I’d dial the cooling nitrogen source back again as one of the micro crystals grew into a single crystal that filled the capillary. So, that’s how I got the single crystals. Actually, I tried unsuccessfully for about three weeks. And I went into Baenziger’s office and asked, “Are you sure this can be done?” He said, “Yes.” So I went back into the lab and did it. I still needed cross data for scaling and found out that single crystal orientation varied with capillary diameter, the same crystal form would crystallize in different directions. I was able to determine the structure. One day I asked Baenziger, “ How did you know it could be done?” He said, “Well. I knew it could be done. I just didn’t know how long it would take you.” [laugh] He had confidence that somehow I would figure it out. There were some stray lines in the pattern. I figured out those were ice lines. That led me to have a better understanding of powder patterns. That helped me when I went to Abe Clearfield to work on the powder of these ion exchange things that were useful to him. But, I strayed, as usual. Your question was really about technological advances. So, we’d go from that stage of measuring the data off of film to right now, any structure up to 150 atoms could be solved in a day. Getting the crystal may be the only remaining challenge. Now there are programs that will write your paper for you. I got to the point where each steroid structure was almost identical in format to all others. But each of the ribosomal proteins will have its own story to tell about its place in the evolution of the ribosome. We have already found that S9 had to be one of the very first ribosomal proteins because it interacts critically with the transfer RNA (tRNA), the messenger RNA (mRNA), and the ribosomal RNA (rRNA) in the process of all protein synthesis. I’ve told my students, “You’re all going to have to determine what role your ribosomal protein plays in ribosome function.” When the first crystal structures of ribosomes were determined it was found that protein synthesis in the ribosome primarily takes place where the mRNA, tRNA, and rRNA come together. This led to the birth of the RNA world. Biochemist concluded that RNA preceded DNA and that the earliest proteins were produced without DNA. Our data does not confirm or refute this. Our data does refute other things, such as textbook assertions that Methionine is the first amino acid in all proteins. My students have proven to themselves, that that is not true. The DNA code for Methionine is ATG (Adenine, Thymine, Guanine). If a protein begins with Methionine its DNA should begin with ATG. The earliest proteins had only a single membrane. After about a billion years of nothing but single membrane proteins, proteins with a double membrane evolved. How that happened is uncertain. We think that our analysis of the evolution of ribosomal proteins may shed light on the nature of that event. The very oldest of the single membrane proteins are the Actinobacteria When we examine the sequences of the coding frame in the DNA of the ribosomal proteins of these ancient bacteria, we find many of them do not begin with the ATG sequence of methionine but the GTG of Valine and CTG of Leucine. Not only do many of these ribosomal proteins in Actinobacteria not have methionine ATG as their start code, they don’t have ATG anywhere in their sequence. These proteins have no methionines. A strand of DNA can be read six ways. They can be read three ways on the coding strand and three ways on the anticoding strand. When we examine the coding strand of ribosomal proteins in Actinobacteria that do not have ATG in the coding frame, many of them do not have ATG in the coding strand. Methionine is not found in these ancient proteins that are encoded by DNA that has no ATG sequences in 5 of 6 possible coding frames. We have evidence that methionine was not always in the genetic code and we can determine whether it first appeared as a start reside or a functional residue in the sequence. We have tabulated the distribution of Valine, Leucine, and Methionine starts in proteins of the small subunit of the ribosomes of all Actinobacteria in the gene bank. We are able to determine how many of the earliest actinobacteria started with a valine, how many started with leucine, what was the real starting signal, and when methionine first appears. If the start code of one ribosomal protein in Actinobacteria is far more often a methionine than the start code of another it may be that the one that starts with methionine more often evolved later than the one with fewer methionine starts. I see you’re smiling again so I think I’ve done that one. ZIERLER: Absolutely. [laugh] No, no, no, no, no. It’s fascinating. No, no. Don’t read incorrectly into that. [laugh] Bill, can you talk a little bit more about the development of the Cambridge Crystallographic Database? In what ways have you gotten, sort of, real satisfaction from the research abilities that this database has created? DUAX: I haven’t used the Cambridge Structural Database very much since we have shifted our focus from steroids to the proteins with which they interact. When we did use the CSD, it was very helpful. The staff at the CSD center have developed excellent programs to analyze the data. Helen says the same is true of the Protein Data Bank. The databanks are one thing. How to use them is another. Bioinformatics people have the skill to develop programs to analyze data, but they may not have the understanding of molecular structure information needed to write programs that use the data in the CSD and PDB most effectively. It’s probably been 10 years since we’ve made use of the Cambridge Database at HWI. But the databases continue to be needed by crystallographers and especially to non-crystallographers. The software that Helen and Olga’s teams have helped non-crystallographers to design drugs and determine molecular function. ZIERLER: Bill, I’d like you talk a little bit about your involvement with the ACA over the years. You know, first as a member of the field and then in your ever growing official capacity. DUAX:I went to ACA meetings very early on. I can remember that one of the people who impressed me at ACA meetings was Isabella Karle. She applied the methods that Herb and Jerry developed and she gave wonderful talks. Someone in South Africa had sent her a sample of a frog toxin. She showed a picture of the ugly frog and gave details of how she determined the structure and talked about how the toxin worked. Isabella developed something called symbolic addition to solve structures. And she taught a lot of people how to use symbolic addition. She spoke beautifully and she taught well. Michael Woolfson and his colleagues wrote a program to automate structure solutions that was called MULTAN. And at the time the Nobel Prize was awarded to Herb and Jerry, there was a lot of controversy as to whether Isabella Karle or Michael Wolfson should share the prize. But the Nobel Prize can only be given to a maximum of three people. I have written editorials criticizing that restriction because it is divisive. That it should not have been limited to three people. It should be whatever is the appropriate number of people to acknowledge for what was done.
Chuck Weeks, Bill Duax, Herbert Hauptman, and Steve Potter the day the Nobel Prize was awarded to Hauptman.
Jerry and Herb were identified as the people who were responsible for the theory. But when you identify the people involved in the theory, there’s a third person who should have been included. That was David Sayre who developed the tangent formula. David also was also one of the developers of FORTRAN David Sayre’s contributions were critical to Herb’s ability to evolve the theory of structure determination. Sayre always called it the tangent formula; he never called it the Sayre equation. The rest of the world called it the Sayre equation. He was hurt when the prize went to Herb and Jerry. And Isabella was hurt. And Michael Wolfson was hurt. And their supporters and followers were hurt. Awarding the Nobel Prize to Hauptman and Karle damaged relationships among their relationship among all of these people. ZIERLER: What was so serious about this? What do you think exactly caused all of these feelings? DUAX: The fact that Herb and Jerry were given this award which seemed to give them full credit for direct methods and seemed to ignore the contribution of others for whom significance of the contribution was unquestioned by the community at large. ZIERLER: Do you think the way that the award was accepted, that the recipients might have done more to emphasize that the collaboration was in fact, much larger than the recognition? DUAX: That would have been a good idea. But that isn’t the way things went until Ada Yonath got the Nobel Prize for the ribosome. ZIERLER: Yeah. DUAX: Ada shared the prize with two other people. When expressing appreciation for being awarded the Nobel Prize Ada Yonath brought her entire team together behind her and said, “These are the people who did this.” She very graciously acknowledged the support of all of the team that worked with her. Ada is a wonderful example of a Nobel Laureate. For years dozens of people tried to grow crystals of a ribosome. When Ada succeeded in growing crystals she told the scientific community how she did it. Soon others had crystals, too. Ada shared the Nobel prize with Venki Ramakrishnan, who was working in Cambridge, England, and Tom Steitz, a Howard Hughes Fellow in the US. Cambridge and the Howard Hughes Foundation invested heavily in the competition to complete the first structure determination. Paul Sigler (MD-PhD-crystallographer) was very concerned that Ada was gonna lose out. Before any structure was completed Ada received a prestigious award for growing the first crystals. Ramakrishnan went on to be the first Indian to be the president of the Royal Society, which is a very good thing. ZIERLER: Right. DUAX: In the U.S., we had strong support from the Indian postdocs, but they were never given a leadership role in the ACA until S. Narasinga Rao was elected treasurer and later became the first CEO of the ACA. That was the first time an Indian scientist was given a significant role in the management of the ACA. Prior to that, when I became president, I asked one of the leading Indian scientists, Muttaiya Sundaralingam if he would chair of a committee to choose the winner of an ACA award. Sundar was very pleased to be asked. I took a lot of pictures at ACA meetings. I took pictures of everybody. Husbands, wives, children, everybody. I would bring copies of the pictures back to the next years ACA meeting and give people their pictures. Paul Sigler said that all the best pictures he had from ACA meetings came from me. I did the same thing all my life with other gatherings. I went to ACA meetings to enjoy the presentations. I went to sessions in which I saw Hauptman and Karle being badgered and ridiculed by David Harker and others until the Nobel Prize turned that all around and people who wouldn’t pay any attention to their research wanted to learn how to do it and the schools for direct methods started. When I was elected president of the ACA, headquarters was at the AIP in New York City. We had a secretary who came in for one day a week to sort correspondence and work on our newsletter. When I was elected, I started a membership drive. Six months later, nobody had applied for membership and I couldn’t understand it. I went to NYC to see what was going on. On our secretary’s desk there was a stack of correspondence that had to be at least a foot and a half tall. On the top of the stack was a letter from someone complaining that they had applied for membership two months earlier and hadn’t heard anything. Further down in the stack, I came across that application. All applications for membership just accumulated on the stack. The secretary would come in each week and look at a few pieces of correspondence on the top of the stack but had too little time to deal with all or even sort it. I returned to NYC a week later and tossed everything off the desk and into suitcases, brought it back to Buffalo, hired a halftime secretary, and went through the correspondence. I persuaded the membership that we did not need to have an office in New York City. People in New York said, “How can you run an office in Buffalo? Out there in the sticks? It’s much better to run it in Washington, D.C. or New York, where we know how to do things.” I have been an invited speaker at national meetings when another invited speaker asked me, “Why are you in Buffalo? And I said, “Well, I have a wonderful opportunity in Buffalo. It’s great. I can do research…” “Yes, but why are you in Buffalo?” When he asked a third time I realized he was saying, “If you are any good why the hell are you in Buffalo?” ZIERLER: Right. DUAX: Baloney. So, I brought the office to Buffalo and eventually got a full-time secretary. And we have worked it out to where we can have a full time secretary. The ACA newsletter was edited by Jenny Glusker and published by the AIP. We started doing the preparation of the ACA newsletter in Buffalo. We still used some of the facilities of the AIP. The AIP was very good at helping us with production and distribution. Advertising was another story. The AIP staff didn’t know our advertisers and our advertisers didn’t know them. All ACA business was all handled by the ACA council. When its members met at the two ACA meetings each year. I was the first person to suggest we have only one ACA meeting each year because we needed to go to other scientific meetings and spread the gospel of crystallography. We might benefit by having one meeting a year where more of the members came together. The council was unhappy because they felt they needed two meetings a year to conduct ACA business. I felt that most ACA business could be handled better by the full time secretary in Buffalo. About four years later when Philip Coppens was president he proposed that the ACA meet only once a year. Unanimous approval. With my help the full time secretary was able to take on all of the administrative activities previously done by the ACA council. Negotiating charges from convention centers, hotels, exhibitors, advertisers, printers, publishers, and excursion sites. Marcia Vair [Colquhoun] was the first full time secretary and she was fabulous. She took on more and more responsibility. Her salary was compensated appropriately, even though she didn’t go to college. Some of the council members were upset that she was getting a higher salary than their secretaries. I had to make clear exactly what she was doing, and they always supported the salary increases that I recommended. But they resented it. So, you learn how to deal with people and how they express themselves. ZIERLER: Yeah. DUAX: With the pandemic, we’ve just gone through the first virtual program and it has been successful. My first virtual high school student summer school was also a success. Previously, I only had students coming from the immediate area, except for one person from Toronto who talked her father into taking a room at a local hotel for three weeks so she could come to the summer school. In this years virtual school we had two students from California, one from Oregon, and two from Boston. The ACA has evolved to where it’s going to hold virtual meetings as long as necessary. After Buffalo became a leader in the US crystallographic community I was asked to be the editor of a newsletter of the IUCr. The IUCr has a large staff in England that is responsible for the publication of all its journals but they did not feel they could take on publication of a newsletter. André Authier, the then president of the IUCr called me and asked if I would edit the newsletter. I agreed to be the editor with AIP as the publisher. Andre had wanted to have it published by Springer. I knew and trusted so many people at the AIP, I knew that Springer was not the partner I wanted to work with. I was on the committee that selected the site for the AIP headquarters outside of Washington. Which was initially, I thought, a good location. But it hasn’t prospered as well in its location as it might have, had it been located more proximate to downtown Washington. Some of the people who originally shared offices in the AIP building have moved into other quarters in downtown. So, the ACA affiliation offered me the opportunity to learn the benefits and the power of the AIP. And the AIP was the right choice to help me get the IUCr newsletter up and running. Eventually it became clear that the AIP was not as capable of raising advertising funds as we could from the ACA office in Buffalo and their advertising service fees would chew up most of the income. AIP continued to do production and mailing for another year, and then with their help, made contact with the people that they use for printing and distribution and began to use them from Buffalo. Managing advertising in Buffalo turned out to be a smart move. We were sending the IUCr newsletter to all the crystallographers in the world. Not only those in the world directory of crystallographers but those on attendee lists of meetings that crystallographers frequented. We had a huge list of addresses of people worldwide that probably included 90% of all the scientists in the world that might buy crystallographic equipment or supplies. Anyone who is trying to sell a diffractometer is going to reach all of their potential buyers by putting an ad in the IUCr newsletter. It was much cheaper to advertise in the IUCr newsletter, than C&EN News, Science and Nature at a much higher cost. The IUCr Newsletter began to cover most of expenses from advertising while partly subsidized by the IUCr as a service to crystallographers everywhere. Eventually the advertisers also knew how to contact potential customers directly and avoid the cost of advertising in magazines. We watched the evolution of advertising and marketing, and tried to keep the ACA solvent. When I became president, the endowment of the ACA was about $250,000. Due to having successful meetings, a hard working office staff, and a treasurer who is financially brilliant, our endowment is now over a million dollars. Which is still only a million dollars, but it’s better than $250,000 and gives us a somewhat greater security. ACA meetings, which were attracting about 200 people in the year I was elected president are now routinely attracting 600 people. The virtual meeting had 600 participants with reduced expenses and income. People are saying, it’s unlikely we would go back to a physical meeting because virtual meetings have more advantages than drawbacks. You avoid the costs of housing, gas, and airfare. Virtual meetings are better for the economy, the climate, and the environment. We just have to make virtual meeting more effective and more appealing. I think that the virtual Democratic Convention had several elements in it that were brilliant. The opening scene where all of these children from across all 50 states finally dissolve into a montage of red, white, and blue stars was great. ZIERLER: Bill, given both all of your work at the national level at the ACA and at the international level with crystallography, in what ways does having both that national and the international perspectives sort of advance crystallography, generally? DUAX: Crystallography’s been international right from its beginning. Very early on there were forty member countries. Over the course of my career, I’ve visited most of the 40 countries. In particular, when I was president, I made a trip to South America because I specifically wanted to touch base with all of the members there. The ACA had begun a South American outreach program to help South America to become a regional affiliate. In addition to the individual 40 country members, there are affiliate members. The ACA, AsCA (Asian Crystallographic Association), and ECA (European Crystallographic Association) are affiliate members. The IUCr has developed many ways to draw crystallographers from all over the world people into their commissions, committees, and national and regional affiliates. We hope there will soon be an African regional affiliate. There was a meeting of the African crystallographers that I attended a few years ago. I am reluctant to go to any more meetings, anywhere. Not just because of the pandemic, but because of my age and because of things that I still want to accomplish. But I would be interested to return to Africa. I feel that it’s an important place to be. When I was president I gave a talk saying that the future of crystallography was going to be in Latin America, Asia, Africa. And I believe that that is the case. Crystallography allows you to maintain intellectual property rights to things in your country. Each country has its own ensemble of plants, insects, and bacteria. US scientists went to Mexico and extracted steroidal compounds from tons of yams. They returned to the US, went to Eli Lilly and used the steroids from the yams to synthesize steroids for birth control pills. If the technology was available in Mexico birth control could have been produced there. This might have benefitted the economy of Mexico and its overpopulation. When I visited the South American countries as president I urged them to expand their technical expertise and preserve your intellectual property rights. As president of the IUCr I advocated gender equity in the membership of IUCr Commissions and Committees. As program chair for the1996 IUCr Congress in Seattle I told the executive committee of the IUCr that my program committee was going to have gender equitable composition. The executive committee of all men told me that that was a mistake. They said I should only decide who’s on the program committee based upon who are the best crystallographers. I assured them that all the women that I would put on the committee would be as bright as or brighter than any of the men on the committee. When I was president of ACA, I took the unilateral action of running two women for president in the next year’s election because women had been underrepresented in leadership positions in ACA for years. Helen Berman and Ann Kerr were the candidates and Helen was elected. ZIERLER: Bill, that’s a great segue. You mentioned, you know, there’s still things that you’re working on currently. Just to bring the narrative up to the current date, what are some of those projects that you’re working on and you have been working on in recent years? DUAX:I strongly support Black Lives Matter. I did not address minority leadership in the ACA or the IUCr as I did gender equity. My wife Caroline and I have four children. Our fourth child is a black that we adopted when he was an infant. At the time of the adoption it was an action that was not well thought out, but an action that has turned out to be very beneficial in the long run. It has sensitized me to what it is like to grow up Black in America. I’ve been wearing this button that says I am anti-racist for about three years now. My son gave me the first one that I wore and then I bought 200 and I’ve given away most of them. When people see me wearing it in a store or on the subway and many say that they like it. I ask if they want one and if they say yes I give them one. One person thought that instead of “racist” it said “radish” and that I was an anti-vegan. ZIERLER:[laugh] DUAX: I’m in two speaking groups in Buffalo that have a 40- and 50-year history. One of them still has only white, male members. It does not allow women or any minorities. There are no bylaws that say that; it’s just a fact. The other group started as all male, but they eventually began to accept women members. They accepted women because they wanted the women to listen to them. At first the women didn’t speak but recently they have been giving excellent talks. Shortly after the Sandy Hook massacre a speakers in the all male group gave a talk and a demonstration of weaponry. He brought about 20 guns into the room for the talk including an AK rifle like the one used to kill the children. Subsequently another person gave a presentation explaining why we needed Donald Trump’s wall. I had to sit through that hour presentation. Afterwards, since I was already identified as the flaming liberal in the group, they turned to me and said, “Well, don’t you have any comments, Bill?”. I made some comment and left the room. I was scheduled to speak the next month, which was February, black history month. I talked about my personal Black history. And described how I was brought up to be prejudiced. To be a white supremist. And how my life experiences caused me to change and turn away from it. How by the time I got to college I was a bleeding-heart liberal. When I married I told my wife I thought we shouldn’t have children. We should adopt children because there were too many unwanted children in the world. She cried and said, “Oh, you don’t want to have children with me.” After she had three children and had morning sickness every day of the pregnancies she decided that adopting was not a bad idea after all. I thought, “Well, I’m having enough trouble raising three kids on my salary.” But she persevered and we adopted my son, Stephen Martin. Every step of the way has been a learning process. Finding out that people who did want to adopt a Black child, there were a portion of them who would only adopt a Black girl because a boy would carry the family name. So, who are these people who will raise a Black child, but don’t want their name to be borne by Blacks? Those are the good people! [laugh]. I’ve sent you a copy of my Black history script. The good Blacks were Aunt Jemima, who cooked you pancakes in the morning and Uncle Ben, who made your rice for you. And all of the other black servants. White supremacists are happy to employ a few black servants as long as they don’t get uppity. I’m very happy with the Black Lives movement. I have gone carefully to a couple of demonstrations. I am not crazy about Biden, but I find Trump to be revolting. [This was taped before the Jan 6 insurrection.] I think Kamala Harris is terrific. So, that’s my political point of view. And I don’t know what I started on and then drifted. ZIERLER:[laugh] Well, you’ve certainly gotten excited about sociological and cultural issues over the past few years. Bill, I think for my last question, I want to ask a forward looking question. And that is, sort of, one that combines all of the many things we’ve talked about over the course of our discussion today. And that is, what are you most personally excited about in the field of crystallography looking to the future? And what are you most optimistic about regarding this historical moment that we find ourselves in with Black Lives Matter and including and increasing diversity and inclusivity in the field of science, generally? What are you most optimistic and excited about looking ahead in both of these realms? DUAX: I think within the crystallographic community, cryo-electron microscopy is the wave of the future. It makes it possible to to study microcrystals and assemblies of three or four molecules that are brought together to function. It is possible to assemble things in a crystal, trigger a reaction and watch the reaction happen within the crystal. We are learning more and more about the dynamic processes. That are observable with crystallographic and electron diffraction techniques. We will continue to expand our understanding of how the world works. I guess my goal has always been to explore the underlying reality around us. I think that virtual meetings are a wave of the future out of respect for the environment. I think most people will come to accept that the world has evolved for billions of years and that God and religion only appeared when the humanoid brain came to be in the last 100 thousand years. And people will realize that an afterlife is a fantasy that becomes appealing at the time of death to those who die and those who love and don’t know how to survive the loss. I’m currently in the process of surviving the loss of my wife and daughter. So, I feel very sensitive to this issue. The U.S. was founded on the basis of white supremacy with Blacks being identified as two-thirds of a human white. The earliest men’s clubs excluded women and non-whites from membership. And were white, male supremacist bastions. Obama’s election was welcomed as evidence of change. But things didn’t really change. We had the backlash of the march in Charlotte. With “good people on both sides”. Minorities are still excluded from membership in many men’s clubs, discouraged from joining, or not welcomed in ways that would welcome them. That is not just the problem of the white community it’s a problem of the white-Black community. We don’t know how to relate to each other. We don’t appreciate each other and we think differently in many ways and feel differently. And we have to learn how the other thinks and feels if we’re going to survive. And, that’s enough. I think I’ve covered it. [laugh] Thank you very much for this opportunity. ZIERLER: Bill, it’s been an absolute pleasure speaking with you. It’s very heartening and reaffirming to hear how your passion over the years has reverberated in so many different areas. In research, in service, in socioeconomic, and cultural issues. And I’m so glad that we connected and I want to wish you a lot of luck in your future endeavors. DUAX: Thank you very much. [End] Postscript: BuffaloMy wife and I have been very active in civic projects in Buffalo. In 1979, my wife Caroline graduated from Daemen College, where she majored in performance piano. The Concord Trio that she formed had local performances including fund raising events for a local dance company in which our daughter Sarah was the youngest dancer. Caroline received a master’s degree from the School of Social Work of SUNY Buffalo (1989) and worked for Catholic Charities from 1989 to 1995. She was on the Family Selection Committee for Habitat for Humanity from 2000 to 2010. We were co-chairs of the Buffalo chapter of American Field Service for two years after our oldest daughter Julia spent 6 months in Tunis. We organized and prepared annual fund-raising dinners for 100 people. In 2002 Caroline collected 700 signatures to preserve the Historic Gate house of the [Arthur] Hedstrom Mansion in Amherst. In 2006 we purchased the property to preserve the gatehouse, barn and stable, half an acre of woods and the stonewall surrounding the property. In 2012 the gatehouse was designated as a landmark by the Historic Preservation Commission and we were awarded the Rehabilitation /Adaptive Reuse Award from the Preservation Buffalo Niagara Society. Caroline served on the Amherst Historic Preservation Commission from 2010 to 2017. The Saturn Club of Buffalo was founded in 1885 and has had the longest running annual variety show of any club in the United States. The 100th Saturn [Club] Chairing [of the Dean] Show will be celebrated next year. I have sung and danced in 27 shows and was the principal librettist for 20 of them satisfying my high school ambitions to be an actor and a writer. |