- History Home
- People, Leadership & Service
- A Legacy of Excellence
- History & Impact
- Meetings Through the Years
- Resources
Memoir - David J. HaasMemoir | Publications | Curriculum Vitae | Videos | Slides | Interviews | Articles | Obituary
ACA Living History
1960-1965. PHYSICS, BIOPHYSICS, CRYSTALLOGRAPHY and FAMILY AT THE UNIVERSITY OF BUFFALO.
I graduated from the University of Buffalo (now SUNY at Buffalo) in 1962 with a physics degree, and then entered the Department of Biophysics in the Medical School. The department head was Dr. Fred Snell who was well known in at SUNY Buffalo as an innovator in molecular biology. Fred Snell created a thriving department with several of his students becoming major scientists. After my departure, Fred became a national leader in the anti-Vietnam War Movement and had major impacts throughout the scientific community (he had been a member of the WWII Atomic Bomb Casualty Commission). The department was closely associated with Roswell Park Memorial Cancer Institute where the Crystallographic Center had been established in 1960. This new center was created to house "The Protein Structure Project" which had been operating in the Crystallography Department of Brooklyn Polytechnic Institute as a guest of Isidor Fankuchen (In 1936, Fankuchen had worked with Bernal and Perutz in Cambridge, so he understood the status of protein crystallography in 1950). Dr. David Harker was provided with a million dollars in 1949 for "The Protein Structure Project" by Irving Langmuir [Harker 1965; Tulinsky 1996; Goodman 1953]. Isidor Fankuchen apparently had sufficient interest, space and facilities for such an organization and invited David Harker to establish it there. The plan was to remain for about ten years and arrangements were made in 1960 to move the entire group to Buffalo. For the first few years in Buffalo, the crystallography group was situated in the basement of the Roswell Park Research Building and then in 1968 the Crystallographic Center was constructed on the Roswell Park Campus.
1965. PhD THESIS AT ROSWEL PARK MEMORIAL INSTITUTE/UNIV. OF BUFFALO.
With Dr. Harker as my advisor, I specialized in x-ray crystallography at the Crystallographic Center where I used the original goniostat units (Eulerian cradle) for collecting diffraction data and superficially participated in the ribonuclease protein work. My thesis project was to solve the crystal structures for six organic molecules which were known protein denaturants. Performing x-ray data collection on the GE goniostats required manual data collection. I spent hours isolated in the x-ray room dialing numbers into these units. All the data was punched into Hollerith cards, and I used one of two IBM 1620 computers which were at the Roswell Park Computer Center and the UB Engineering Department. There were boxes and boxes of punched cards to transport for the structure determination. All the software programs were supplied by Dr. Ahmed in Ottawa, Canada which proved to be essential to my success. (Thank you Dr. Ahmed!) After solving the structures of the six organic molecules in a matter of months and providing some assistance to the ribonuclease group, each of the crystal structures was published as a paper in Acta Crystallographica which presented me with much needed exercises in scientific writing and drawing electron density maps/structures by hand. I can say that Dr. Harker was surprised at how fast I determined these structures, and he instructed me to write my thesis, including all the data that I had sent for publication. I graduated in February 1965.
David Harker (1906-1991).
(AIP Emilio Segre Visual Archives, Physics Today Collection)
David Haas collecting diffraction data with the original Harker-Furnas Goniostat at Roswell Park Memorial Institute (1964).
During this time interval, I met my wife, and we married on June 10, 1962. Sandra and I have been partners ever since! She had unforeseen skills and talents which were demonstrated beyond her raising our three sons, as Vice-President of Temtec, Inc. - the business that we operated for 21 years (1981-2002). She excelled as the HR and customer service manager.
Sandra F. Haas (2000).
1965. SHORT POSTDOC AT THE NAVAL RESEARCH LABORATORY.
Meanwhile, Dr. Harker had contacted several British protein crystallographers to locate a postdoctoral position for me. This was already 5 years after the structures of myoglobin and hemoglobin had been determined, and David C. Philips had just finished with the structure of lysozyme (March 1965). We knew it was an exciting time in Britain for protein crystallographers. David Phillips responded that he would have a postdoc position available for me after September 1965. I immediately accepted. Dr. Harker suggested that in the intervening months I should learn as much as I could about the new direct methods that were just being perfected by the Karles in Washington. Jerry Karle invited me to his lab for several months, so between February and August 1965, Sandra and I rented an apartment near the Naval Research Laboratory for this short interval of study and work. Isabella and Jerry Karle were really wonderful, and I attempted to learn as much as I could about direct methods during this stay. I worked with a research assistant S. A. Brenner on collecting data on dimethylmalonic acid, and we published a paper using Symbolic Addition Methods on the structure [Haas 1966]. Isabella and Jerry were always available for discussions so my time with them was quite beneficial. Also, during the summer they frequently had barbeques for the laboratory staff and their spouses at their home in Falls Church. Great comradery! 1965-1966. POSTDOCTORAL FELLOWSHIP AT THE ROYAL INSTITUTION OF GREAT BRITAIN.
Sandra and I arrived in London September 1965. We spent a few days at David Phillips' home, getting oriented and learning the language! I spoke at length with my officemate - Dr. Charles Bunn who was a well-known industrial crystallographer. The real excitement at the Royal Institution had been the March Friday Evening Lecture by David Phillips on the structure and function of lysozyme, the first enzyme structure to be determined. (He was now working on the Scientific American article.) Even though I was not present to hear it, the culmination of years of work on the lysozyme crystal structure made everyone very happy and now they were looking forward to follow-on work. The various people with David Phillips were now looking for academic positions and several of them discussed the ongoing problem of radiation damage to the crystals. It was suggested that I might investigate the effects and possible uses of crosslinking protein crystals to increase mechanical stability and reduce radiation damage. No other experiments on reducing radiation damage had been successful.
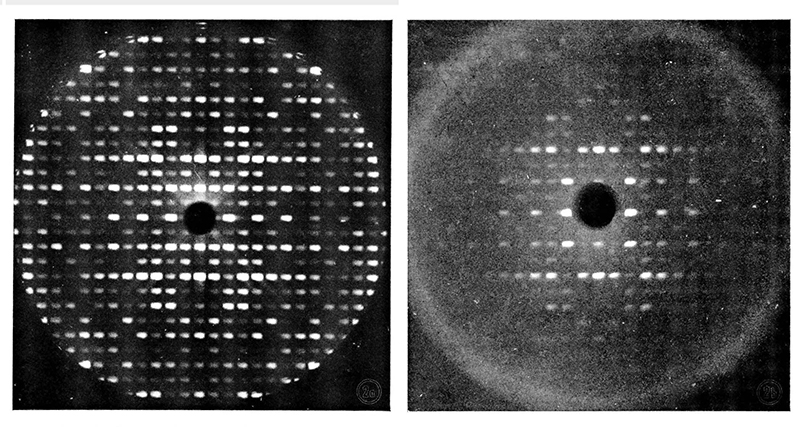
With an ample supply of surplus lysozyme crystals from David Phillips, I first tested crosslinking to different degrees, and lysozyme was an excellent test model as the crystals were very stable and rugged. A measure of the degree of crosslinking was to denature the crystal and observe the volume of swelling. The less the crosslinking, the greater the swelling. For example, brief exposure to a dilute solution of glutaraldehyde produces only a "surface crosslinking". These crystals, when denatured, swelled to enormous sizes, each crystal edge increasing more than three times. I compared this surface crosslinking vs. 100% cross-linked crystals and found that the "surface-crosslinked" crystal would remain unchanged and basically normal. Most important, with denatured surface crosslinked lysozyme crystals, slowly removing the denaturant and returning the crystal back to its original supernatant caused the crystal to shrink again and recover its x-ray diffraction pattern. This proved to be a remarkable renaturation property which shows that the protein molecules could actually recrystallize themselves. I presented several papers at European crystallographic meetings and published a short note in Acta Crystallographica in 1968 [Haas 1968].
Swelling of denatured cross-linked protein crystals. [Haas, 1968].
One of my fondest memories about working at the Royal Institution was the daily 4 p.m. tea gathering. Most of the staff appeared for the daily discussions (Gareth Mair, Colin Blake, Louise Johnson, Tony North, Ragupathy Sarma) including Sir William Lawrence Bragg. I recently read in Georgina Ferry's book on Max Perutz that these tea breaks were begun by Sir William Henry Bragg when he first took over management of the Royal Institution laboratory in 1923.
Precession photographs of native and renatured lysozyme crystals showing
1966-1967. POSTDOCTORAL FELLOWSHIP AT THE WEIZMANN INSTITUTE OF SCIENCE.
In the fall of 1966, David Philips informed me that he was moving the group to Oxford University and that I would need to find another laboratory to continue my NIH Fellowship. He suggested the Weizmann Institute in Israel and arranged for me to work in the Crystallography Group of the Chemistry Department with Wolfie Traub and Gerhard Schmidt. Until recently I had no idea why he made this suggestion, but it appears that he attended a meeting in Israel earlier in 1966 and was impressed with the quality of the people and work at the Weizmann Institute of Science. Wolfie Traub indicated that we should just take a ship to Haifa, and they would be ready for us at any time. As my NIH Fellowship was not transferable, Wolfie said the Weizmann Institute would also provide a Weizmann Fellowship (Thank you Mr. and Mrs. Van Leer for your Fellowship) which included housing and a stipend. The Weizmann Institute proved to be a remarkable scientific institution, and what an experience for both Sandra and me.
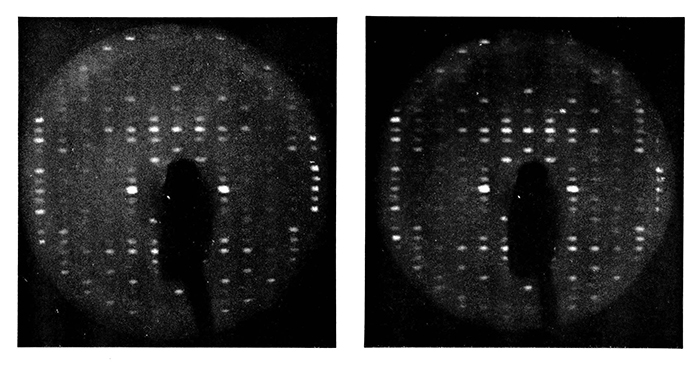
Sandra and I arrived in Israel from England at the Weizmann Institute of Science in December 1966 with our 3-month-old son Stuart. Taking all my lysozyme materials and chemicals, I continued work on the effects of crosslinking protein crystals. I determined that crosslinking had little or no effect on reducing radiation damage by exposing various crystals for days in the full-power x-ray beam and then taking precession photographs of a standard pattern. This was quite disappointing! Fortunately, there was an unused crystal freezing apparatus setup on one of the Philips x-ray generators. Considering whether crosslinked crystals would remain intact when frozen, I determined that crosslinked crystals fractured from ice crystals in exactly the same manner as native (uncrosslinked) crystals. Then I discovered that crosslinked crystals could be put into almost any solution of salts or organic solvents (cryoprotectant) because the crosslinking prevented the crystal from dissolving in the supernatant (as well as providing structural stability). With surface crosslinked lysozyme crystals in different concentrations of sucrose, frozen crystals gave excellent diffraction patterns above a certain sucrose concentration (sufficient to prevent ice formation). I confirmed that freezing in sucrose did not damage the crosslinked crystal by taking standard diffraction patterns repeatedly before and after diluting out the cryoprotectant.
Precession photographs of (LEFT) the first successful frozen protein crystal, crosslinked lysozyme;
Then I discovered that lysozyme crystals could have their supernatant slowly changed to the appropriate compound concentration "without crosslinking" simply by selecting the appropriate compound (at room temperature and taking more precession x-ray patterns). These frozen native crystals in sucrose cryoprotectant gave excellent diffraction patterns, and I was quite impressed with their stability. (Note: The term cryoprotectant had not been created at this time.) Exposing several crystals to many days of x-rays at full power while taking repeat precession patterns demonstrated that the frozen lysozyme crystals showed substantially less radiation damage than native lysozyme crystals at room temperature. Hence, a solution to the radiation problem may be at hand! [Haas 1968]
I was unable to continue this work at the Weizmann because the Six Day War began on June 5, 1967, and I had sent my wife and newborn son back to London for safety. It appeared that I would not be able to resume any serious work for many months, but I had already taken sufficient precession patterns for publication to demonstrate that radiation damage is absolutely reduced by freezing protein crystals! Fortunately, I had already arranged for a position with Michael Rossmann at Purdue when I returned to the United States, so I decided to return in July since most of the Weizmann staff was in the IDF (Israel Defense Forces) and little research would be accomplished for many months.
Working at the Weizmann Institute was exciting as there were regular lectures and events on the campus. I should note that I have recently learned that the cryogenic equipment I used there had been constructed by Wolfie several years earlier for his work, but was then idle, yet in perfect working condition. One of the crystallography research assistants taught me how to use it. If not for this fortuitous event and serious scientific assistance, the proof of "reduced radiation damage" by freezing macromolecular crystals would have been delayed for years. And my follow-on work with Michael Rossmann may not have taken place because Michael was only convinced by the precession x-ray photographs that I repeatedly held in my hand!
1967-1970. ASSISTANT PROFESSOR AT PURDUE UNIVERSITY.
The Rossmann laboratory was a beehive of activity with M. J. Adams, A. J. Wonacott and A. McPherson all working on the lactate dehydrogenase project. Michael had the latest equipment and the group was always willing to help me learn the new methods. Within the first months, I wrote the short paper describing my findings of reduced radiation damage at low temperatures and presented my finding to the Rossmann crystallography group [Haas 1968]. They were skeptical that the benefits of extending the useful life of their crystals would be worth the "perceived" complexity and difficulty of building, installing and operating cooling apparatus. I believe that everyone expected the radiation damage reduction to be only a few hours, not the hundreds of hours that finally came to be with low-temperature x-ray data collection. And certainly, no one could even imagine the importance of freezing crystals with synchrotron radiation sources! Again, I would not have had a convincing argument for reduced radiation damage without the lysozyme precession photographs from the Weizmann Institute. The general notion at the time was that freezing protein crystals was no different from freezing food - I do not believe anyone in the laboratory knew the Birds Eye frozen food story, and it appeared that freezing anything, in general, was a bad idea!
After extended deliberations, Michael Rossmann agreed to loan me one of the Picker Automated Diffractometers, and he would fund the purchase and construction of the most primitive freezing apparatus, as it probably would only be used once for my project. (Thank you Michael!) The glass laboratory fabricated a co-axial gas delivery jet for directing the nitrogen gas onto the crystal with a dry air cylindrical barrier jet surrounding the nitrogen stream. The crystal would be mounted in a Lindemann glass vial in the conventional manner along with a small amount of mother liquor. The work would be performed on lactate dehydrogenase as this was the protein being worked on by Michael Rossmann and sufficient crystals were available. The same sucrose cryoprotectant was successfully used as demonstrated in my previous experience with the lysozyme crystals. Several crystals were prepared and they proved that sucrose would be satisfactory.
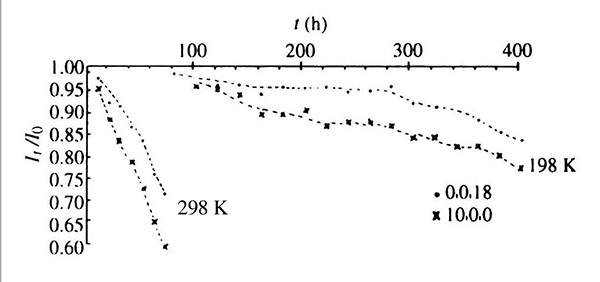
I assembled all the equipment and fabricated simple control circuits, purchased a suitable air dryer and several bathroom scales for tracking the weight on the nitrogen-filled Dewars (the scale weight would show me when the Dewars were running low and they had to be manually refilled). After determining the pounds/hour usage of the liquid nitrogen, I would calculate when the "feed" Dewar had to be manually refilled from the storage Dewar. Of course, this was typically in the middle of the night! I would have to stop the x-ray data collection during refilling since the nitrogen flow rate would fluctuate and change the crystal temperature. The data collection required several crystals due to ice collection on the vial and mistakes. Crystals warmed to room temperature with several failures of the cooling system during the project, and the diffractometer broke down several times (usually because of the rotary mechanical encoders). After more than three months, the data collection portion of the project was complete and we processed the data.
Cryoprotected LDH crystals show less radiation damage at 198 K than unprotected crystals at 298 K.
In particular, I scheduled regular monitoring of two reference reflections so as to measure the actual x-ray deterioration during the hundreds of hours of exposure. The results of this first x-ray data collection from a frozen crystal were published [Haas 1970]. I also presented a paper at the next ACA meeting in 1969. I can only say that there was basically "no interest" in this work whatsoever! Furthermore, I never heard a word about freezing protein crystal for the next 30 years until 1999 when I again visited Michael Rossmann at Purdue. He said, "What a wonderful technique cryocrystallography had become". Actually, I could hardly remember the work, so Michael gave me a copy of our 1970 paper to read!
After attending the 2015 ACA Annual Meeting in Philadelphia, learning about the 100,000 macromolecular structures in the PDB, and reading Jim Pflugrath's paper on "Practical Macromolecular Cryocrystallography" (2015), I have had a real "Back To The Future" experience!
Soon after, I joined industry (Philips Electronic Instruments in Mt Vernon NY), ending my protein crystallography involvement and within two years, ending my work in x-ray crystallography altogether. As a side note, during 1969, I developed an instrument for setting the dihedral angles on the metal atomic models, and this made it very easy to accurately fabricate the backbone for known protein structures. A manufacturer became interested in "Dihedral Angle Dialer" and manufactured it for several years. What is interesting is that I sent a request to the Purdue University Intellectual Property Committee for permission to apply for a patent, but they just had no interest in scientific patents. And I mean NO INTEREST! I recently found their letter of refusal. And now I can note that if I had applied for a patent on the process for freezing protein crystals for radiation damage reduction, they surely would also have refused to consider it! Scientific patents were just for science, not commercialization! How the world has changed! 1970-1983. PRINCIPAL X-RAY SCIENTIST AT PHILIPS ELECTRONIC INSTRUMENTS.
In 1970 I entered industry by joining Philips Electronic Instruments in Mt. Vernon NY as Principal X-ray Scientist and was immediately appointed Radiation Safety Officer. (I now know that being the RSO was considered by the managers as an awful waste of time!) The Mt. Vernon facility was the original Philips plant set up in the United States by N. V. Philips in 1935. The facility manufactured analytical x-ray equipment and sold industrial x-ray systems as well as other Philips instrumentation. I remained on the ANSI Committee for X-Ray Analytical Instruments for many years, but 1977 was the most valuable as we published the revised X-ray Safety Standard N43.2-1977.
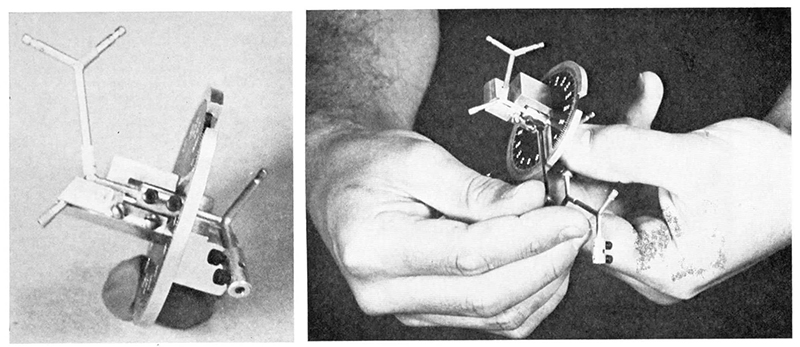
Frustrated before computer model-building graphics, in 1969 I developed this simple instrument for setting the dihedral angles on atomic models. They were sold under the name "Dihedral Angle Dialer" [Haas 1970].
As the Radiation Safety Officer for Philips Electronic Instruments in 1970, I participated in the early development of radiation protection standards for the ANSI Committees for Analytical X-ray Equipment (ANSI N43), Industrial X-ray Equipment (ASTM) and Security Low-Dose Scanning Systems (ASTM F12).
My first project at Philips Electronic Instruments was to complete the software and write the instruction manual for the Automatic Powder Diffractometer, the first computer controlled x-ray diffractometer [Jenkins 1972]. My first project was to complete the software for the Automatic Powder Diffractometer System which I believe was the very first commercial computer-controlled x-ray diffractometer system. It used the Philips Digital Controller (4k with optional 4k for analytics) and would scan 35 consecutive powder samples without human intervention. The automatic sample changer and strip chart recorder/paper tape data output would keep an industrial laboratory fully operational on a continuous QC and analysis basis. After completing the software, I wrote the instruction manual and provided technical expertise for the salesmen and at trade shows. As a high-speed automated instrument, it was very advanced for the early 1970s and many were sold to industry worldwide.
In 1974, I participated in the US Bureau of Radiological Health safety film -
About 1974 I participated in making the analytical x-ray safety film for the Bureau of Radiological Health ("The Double Edged Sword"). The film provided safety advice and precautions for individuals who work with analytical and x-ray spectroscopic equipment. The film appears to have been successful, and I was told that it has continued to be shown into the 21st century. (I recently saw it on YouTube!)
Philips Electronic Instruments was a major innovator and supplier of airport x-ray screening systems for the first decade of aviation passenger screening (1973-1983). As Principal X-ray Scientist, I wrote many articles and became the technical spokesperson at aviation/security meetings and tradeshows [Haas 1976]. In 1973, I was asked to assist in designing the first Philips "Dynafluor" Airport X-ray Security System because on January 5, 1973, the FAA had instituted mandatory aviation passenger security screening: walk-through metal detectors for people and low-dose x-ray for baggage and hand-carried articles. (Several engineers at Philips Government Systems, Mahwah NJ, were the inventors of the original low-dose security system in 1970 [Haas 2010]). During 1973 and 1974, the Philips Industrial Group initially built a lead-lined moving-tunnel unit and then supplied a large conveyorized low-dose x-ray unit to several airlines (Dynafluor IV) that proved to be a "dinosaur". I initiated a stealth project to create a lightweight, mobile, low profile system that provided a successful second-generation system for many airlines (Dynafluor VI). In addition to selling hundreds of units worldwide, the Dynafluor VI provided a modern Electronic Security Screening System similar to the current airport units with low profile, short tunnels, high speed and easy-to-use. So during the 1970s, Philips became a major supplier for aviation security equipment. About a dozen patents were issued for the various Dynafluor Security Systems but Philips terminated the business before enforcing any of their patents.
The 1970s were the formative years for the process called "Electronic Security screening", as this early airport screening operation shows. The design of walk-through metal detectors, low-dose x-ray systems and primative explosive detectors was in constant flux until about 1980 when most equipment went digital (and equipment designs were "standardized" as "human-factor" best practices were established). Electronic Security Screening saved many lives and basically saved the aviation passenger and worldwide tourism industries from disaster [Haas 2010].
A milestone for "Electronic Security Screening" came in 1976 when the Secret Service rented equipment for use at both the Democratic and Republican National Conventions. This was the first public "non-airport" use of the screening equipment. Because Electronic Security Screening was demonstrated by the television networks to the entire United States and worldwide populations, it became accepted by the public and was instituted as "best practice" by the professional security industry [Haas 2010]. At this time, besides being a member of the ANSI F-12 Committee for security screening equipment standards, I was a member of the Photographic Standard Committee addressing the damage problem to photographic and other media materials (magnetic tapes, discs, etc.), with Kodak being the prime mover for low-dose exposure standards and testing. Photographic film damage remained a serious problem from 1973 for several decades until all low-dose systems worldwide were converted to digital imaging.
Philips Electronic Instruments moved to Mahwah, NJ in 1976. I remained with them until 1983 and was mostly involved in low-dose x-ray security matters. For the security professionals of ASIS International (originally the American Society for Industrial Security, basically the worldwide professional security society), I wrote a number of articles and was a regular speaker at security seminars. (Note that Electronic Security Screening was completely new for the professional security industry during this time.) One surprising item was that until 1976 neither the FAA nor any airline had actually provided a visual training program (slides or movie) for security personnel (guards) who operated the metal detectors or low-dose x-ray systems at the airports. Philips decided that this was important (as well as an excellent marketing tool) so we arranged to rent a truck, place a low-dose x-ray unit with an electric generator inside so that we could photograph the actual x-ray images of guns, explosives and bombs. I did this on an Army firing range in Virginia with a substantial variety of explosives and weapons. These high-quality photographic images of actual explosives provided the FAA with an excellent slide-training program for aviation security personnel. The slide set was used for years by airlines security managers all around the world and probably saved many lives because it provided actual x-ray images of guns, bombs, and other hazardous items.
The introduction in 1976 of the Dynafluor X by Philips Electronic Instruments created the modern characteristics for airport Security X-ray Systems: low profile so people could see over the top, very short tunnels with in and out conveyors for high-speed loading and unloading, easy operation and viewing of the X-ray image, light weight, mounted on wheels so they could be moved around airports, and a full x-ray imaging of baggage.
1981-2002. ESTABLISHING TEMTEC INC. (TEMPbadge).
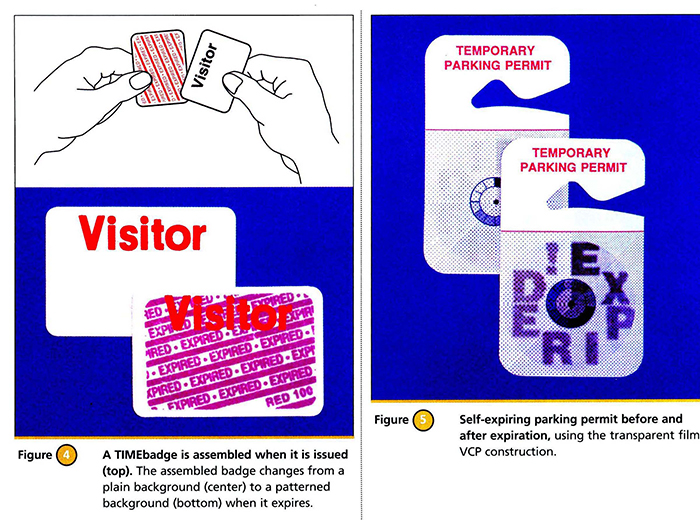
During the late 1970s, I developed an idea for a new type of security ID badge for Visitor Control which would solve the age-old problem of controlling (preventing the reuse) of temporary ID badges (for visitors, contractors, etc.). After several years of laboratory work at home, Sandra and I introduced the self-expiring Visitor Badge which changed color within a day after being issued, to prevent reuse. Our first badge was a coated paper badge with a photochemical that turned blue when the individual left the facility and entered daylight. It was sensitive only to short ultraviolet light and was an effective daytime temporary ID. However, our second self-expiring badge changed color by dye migration and was a one-day Time Expiring Badge. The construction was an adhesive label frontpart applied to a red printed backpart, and in a day, diagonal red strips (or the words EXPIRED) appear which invalidates the credential. This time expiring badge became very successful as a visual self-expiring Visitor Badge, effectively solving the problem of reuse and collecting temporary IDs. Under the brand name of TEMPbadge, we manufactured many types of security ID products. Between 1981-2002, more than 20% of all visitor badges issued in the United States were TEMPbadge badges. The business was sold in 2002. Sandy and I remained at the business for only a few years, but we have remained associated with the security industry ever since [Haas 1998, Haas 2001].
The invention of Self-Expiring Visitor Badges solved a major physical "security-control" problem for professional security managers. Our company Temtec Inc. provided tens of millions of temporary ID badges each year [Haas 1998; Haas 2001; US Patents 5,903,254; 5,058,088; 5,364,132].
The time expiring (chemical) badge is based on the rate of dye migration through a white, opaque adhesive layer. The badges are supplied as two parts, and when the white front part is adhesively attached over the back part at the time of issue, dye migration is initiated. Within a day, the white badge turns red and is "visually" expired [Haas 1998; Haas 2001; US Patents 4,903,254; 5,058,088; 5,364,132].
2002 - PRESENT. NEW PROJECTS.
After 2002 I worked on a number of projects that had been of interest for some time, and we have remained active to this day. In particular, we have returned to the Weizmann Institute of Science several times and supported them with a graduate student fellowship, exactly the same kind that I received in 1966. We were very pleased when Ada Yonath received the Nobel Prize in 2009 for the ribosome structure, using cryocrystallographic techniques. As it turns out, she was a graduate student in the Weizmann Institute's Crystallography Department while I was there in 1966.
After selling Temtec Inc. in 2002, we revisited the Weizmann Institute of Science several times and provided a fellowship for graduate students, like the one provided for me in 1966.
We participated in the Institute's philanthropic activities, which are an example of how young scientists and scientific research can benefit mankind.
One of my desires was to become involved in historical artifacts before they reached the museums, so between 1995 and 2005, I volunteered for many archeological digs and research projects in Israel, Great Britain and Canada. These have provided an ongoing excitement for Sandra and me, particularly when we visit one of the dig sites, and I can show people the inch or so in the "square" that I dug through!
During 2006, I became involved in a short research project for the Smithsonian Institution on the official biography of James Smithson. The author Heather Ewing had begun researching this book in 2000 and because of a publication deadline of June 2007, was unable to research the archives of the Royal Institution of Great Britain on Albemarle Street (the Institution where the Braggs had been directors, the Institution in which James Smithson had been a founding member, and the Institution where I spent 18 months of postdoctoral work in 1966 with David Phillips). With a letter of introduction from the "Historian of the Smithsonian Institution", I spent three week in the Royal Institution's vault looking through all the original minutes and notes from 1799 to 1829, the year that James Smithson passed away. My notes and digital photos of the relevant pages were passed on to Heather Ewing who published the book in late 2007 (The Lost World of James Smithson by Heather Ewing 2007). Her book is now the official biography of James Smithson for the Smithsonian Institution.
In 2006 I volunteered for some Smithsonian research of the early documents at the Royal Institution for the author Heather Ewing (she was writing the Smithsonian's official biography of James Smithson) [Ewing 2007].
I always wanted to know who invented the first photo ID badge, and writing the book Personal Identification: Its Modern Development and Security Implications provided all the answers [Haas 2009].
Beginning in late 2005 with a book project, I began three years of archival research to determine the people instrumental in developing secure identification credentials and in particular, who invented the original photo ID badge. This basically began with the introduction of the passport booklet (about 1875) and made great strides during WWII. Serious advances occurred with the development of the Polaroid camera and the introduction of the photo driver's license in 1959. Originally published in 2009, Personal Identification: Its Modern Development and Security Implications continues to be published through the "worldwide" professional security association ASIS International.Basically, the intent of the book was to answer the question: Why individual citizens had no IDs a century ago, yet today they are essential for every citizen (of almost every country). The book is still available from ASIS International and Amazon.
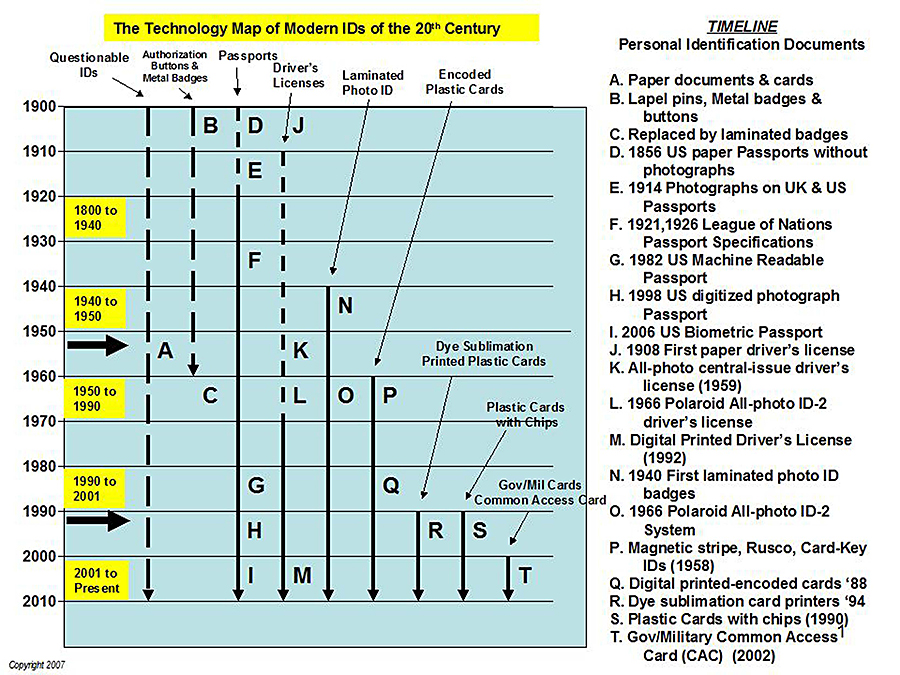
This timeline "Technology Map of Modern ID" demonstrates the impact of the industrial revolution we are living through [Haas 2009].
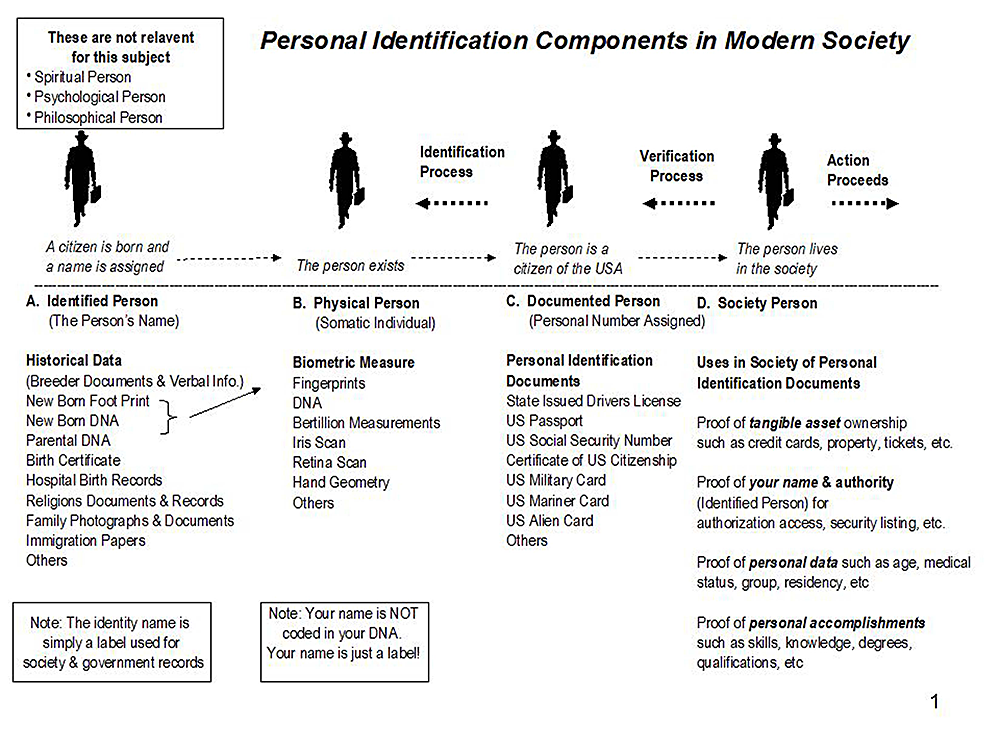
Surprisingly, there is substantial confusion about the actual components of Personal Identification.
During 2007, another project of interest was the invention of the first low-dose security x-ray system, and how the United States Government (and the public) were convinced to pass laws requiring 100% mandatory physical screening of aviation passengers. Within months of my arrival at Philips Electronic Instruments in 1970 I was asked to determine the x-ray exposure of this very first low-dose system at Philips Government Systems in Mahwah NJ - The Saferay. "Little did I know then about the future of this invention." I began several years of archival research and published a book-like article (75 pages) on how and why the first Electronic Security Screening system was invented for aviation passenger security. The walk-through-metal detector had been developed by an electrical engineer in Wilmington, Delaware (Bernard Schwartz) for the FAA in 1969, while the first low-dose x-ray system was developed by an engineering group at Philips Government Systems in Mahwah NJ in 1970 by George W. Shepherd Jr. (deceased) and Neal Diepeveen. The Philips group worked "as an unauthorized project" for three years and demonstrated their system to government and airline officials on Sept 25, 1970 in a hanger at Washington National Airport. This was sufficiently convincing for the airlines and the United States Government to institute and pass laws for mandatory security screening of aviation passengers. With 100% mandatory physical screening of all aviation passengers begun on January 5,1973, the passenger aviation industry was basically saved from worldwide terrorism and criminals. And two new industries were created: metal and explosives detectors for people and low-dose x-ray for baggage and hand-carried articles. The article was published in 2010 [Haas 2010]; George W. Shepherd Jr. and Neil Diepeveen were nominated in 2015 for the National Medal of Technology and Innovation.
In 2010, I published this 75 page article (actually a book) on the two engineers at Philips Government Systems who actually developed the first practical low-dose x-ray system. In 1970, they convinced the United States Government that Electronic Security Screening was practical and would solve the growing aircraft hijacking problem! [Haas 2010].
I believe you will find the abstract on the development of Electronic Security Screening interesting [Haas 2010].
Photograph of the original "Saferay", the first low-dose x-ray system development at Philips Government Systems
Once the US Government passed laws to institute 100% mandatory physical security screening of aviation passengers, the Philips group offered this first practical airport system. Both manually loaded and converized systems were designed by the Saferay group [Haas 2010].
In 1999, after becoming aware of the success of biological crystal structure determination, I have once again begun reading, watching and attending crystallographic lectures as well as ACA meetings. The entire field of cryocrystallography is new to me, but Elspeth Garman provided me with a number of technical articles on the subject. WOW! In 2006, I attended the cryocrystallography session held at the ACA meeting in Chicago and have been watching many historical lectures on the internet (like the ACA historical videos). I am amazed by the enormous technical growth in biological structures determination in the past 40 years. Crystallography has really become an extraordinary research and industry tool for society!
References
BRH Double Edged Sword; National Bureau of Standards, Bureau of Radiological Health 1976 - 30-minute movie on Radiation Safety.
Ewing, Heather. The Lost World of James Smithson; Bloomsbury USA, 2007.
Garman, E.F.; Schneider, T.R. Macromolecular Cryocrystallography. J. Appl. Cryst. (1997) 30, 211-237.
Goodman, David. Protein Structure Project. The Scientific Monthly, Vol. LXXVII, No. 2 Aug 1953.
Harker, D. Cancer Researchers Believe Protein Molecules May Hold Key To 'Secret Of Life'. X-ray Diffraction News, General Electric Company, Milwaukee WI, June 1, 1965.
Haas, D.J.; Brenner, S. A. Application of the Symbolic Addition Method to Dimethylmalonic Acid. Acta Cryst. (1966) 20, 709.
Haas, D.J. X-Ray studies on Lysozyme crystals at -50 C. Acta Cryst. (1968) B24, 604.
Haas, D.J. Preliminary studies on the denaturation of cross-linked lysozyme crystals. Biophysical Journal (1968) 8, 549.
Haas, D.J.; Lentz, P.J. A simplified method of constructing skeletal models of proteins. Biopolymers (1969) 7, 809.
Haas, D.J.; Rossmann, M.G. Crystallographic Studies on Lactate Dehydrogenase at -75 C. Acta Cryst. (1970), B26, 998.
Haas, D.J. Polypeptide & Carbon Models: a new Concept in Protein Model Construction. Biopolymers (1970), 9, 1547.
Haas, D.J. The New Low-Dose X-Ray Machines. Security Management, January, 1976, pp. 14-18.
Haas, D. J. Now Practical - See Through Security. Security World, August, 1976, pp. 20-24.
Haas, D.J. The Technology Behind X-Ray Security Systems, Pro. Soc. Photo-Optical Inst. Engr. 78, March, 1976, pp. 44-47.
Haas, D.J. Self-Expiring IDs and Security Indicators. Cowise Conference, March 18-19, 1998.
Haas, D.J. Time's UP! The Color-Changing Self-Expiring Badge. Chemical Innovation Magazine, 31(2), February, 2001.
Haas, D.J. Personal Identification: Its Modern Development and Security Implications; ASIS International: Alexandria VA, 2009.
Haas, D. J. Electronic Security Screening: Its Origin with Aviation Security 1968-1973; J. Applied Security Research 5, 460-532, 2010.
Jenkins, R.; Haas, D.J.; Paolini, F.R. A New Concept in Automated X-Ray Powder Diffractometers. Norelco Reporter,18(2), 1972.
Jenkins, R.; Haas D.J. Hazards in the Use of X-Ray Analytical Instrumentation. X-Ray Spectrometry 2, 1973.
Tulinsky, A. The Protein Structure Project 1950-1959. In Annual Reports in Medicinal Chemistry, 31, Chapter 35, p 357, 1996.
US Patent 4,903,254. Feb 20, 1990. TIME INDICATOR ENHANCEMENT METHOD.
US Patent 5,058,088. Oct 15, 1991. TIME INDICATOR.
US Patent 5,364,132. Nov 15, 1994. METHOD FOR ASSEMBLY AND ACTIVATION OF A REUSABLE SECURITY IDENTIFICATION BADGE. |